Boron: Uses, Borides, Boranes, Boron Trihalides, Boric Acid (11/20)
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
is boron a metal?
no
Describe the structure of B12
icosahedron
What is an icosahedron?
20 faces, 30 edges, 12 vertices
is boron easy to purify?
no
why is boron difficult to purify?
high MP, corrosive liquid
easier method for purifying boron?
can reduce B2O3 by Mg, resulting in 98% pure powder
harder method for purifying boron?
boron halide + reducing agent + catalyst → pure boron + compound
B2O3 / B(OH)3 are ___ (acidic/basic/amphoteric)
acidic
Borates/silicates have ___ structures
similar
B/Si halides are ___ hydrolyzed
readily
B/Si ___ are volatile/flammable/hydrolyzed
hydrides
name two uses of boron
pyrex, cleaning agents
describe thermal shock
when something is heated to a high temperature and then immediately cooled, the immediate attempt to shrink can cause breakage (ex. glass)
why is pyrex better than glass?
has more thermal shock resistance
what structure is pyrex similar to?
SiO2
describe boride structure
hard, high MP, chemically resistant
what boron compound is used in armor & bicycle frames?
boron carbide
how do you make boron carbide?
reduction of B2O3 with C
describe the structure of titanium boride
hexagonal sheets of boron ions, each boride has -1 oxidation
how do you make titanium boride?
reduction of TiO2 with C in presence of B4C
is magnesium boride good at conducting?
yes - superconducts usually
describe boranes’ bonding
electron deficient
draw the diborane structure
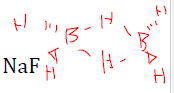
Describe diborane
highly reactive, toxic, colorless gas
borane + moisture =
boric acid, dihydrogen
diborane used in ___ of organic compounds
reduction
what are the five class structures of borane?
closo, nido, arachno, hypho, conjuncto
describe the polyhedron of closo borane
complete, regular
describe the polyhedron of nido borane
(n+1) vertex polyhedron
describe the polyhedron of arachno borane
(n+2) vertex polyhedron
describe the polyhedron of hypho borane
(n+3) vertex polyhedron
Wade’s Rules: closo
F = 2n + 2
Wade’s Rules: nido
F = 2n + 4
Wade’s Rules: arachno
F = 2n + 6
what type of borane structures might see a B-H-B bridge bond?
nido, arachno
what type of borane structures might see a B-H terminal atom?
nido, arachno
what type of borane structures might see a BH2 group?
arachno
B4H10: give the borane nomenclature
tetraborane(10)
B3H7: give the borane nomenclature
triborane(7)
give the equation for Wade’s Rules
F = 3b + 4c + h + x - 2n
What kind of acids are boron trihalides?
Lewis acids
Why are boron trihalides Lewis acids?
vacant p orbital?
If an acid has more overlapping of p orbitals, it is ___
weaker
Give the orbital mixing explanation for why BF3 is a weaker acid than BBr3.
more overlapping = more orbital mixing is more favorable with a good energy max (more likely to get this with 2p F then a 4p Br orbital)
Give the bond distance explanation for why BF3 is a weaker acid than BBr3.
smaller halide = less bond distance = more overlapping = weaker acid
What type of bond character do we see in BCl3?
single
What type of bond character do we see in BF3?
single, double
What kind of acid is boric acid?
Lewis acid