group 1 and 17 elements
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Metallic character
Tendency of an element to lose electrons to form positive ions
elements lower left of PT = greatest metallic character
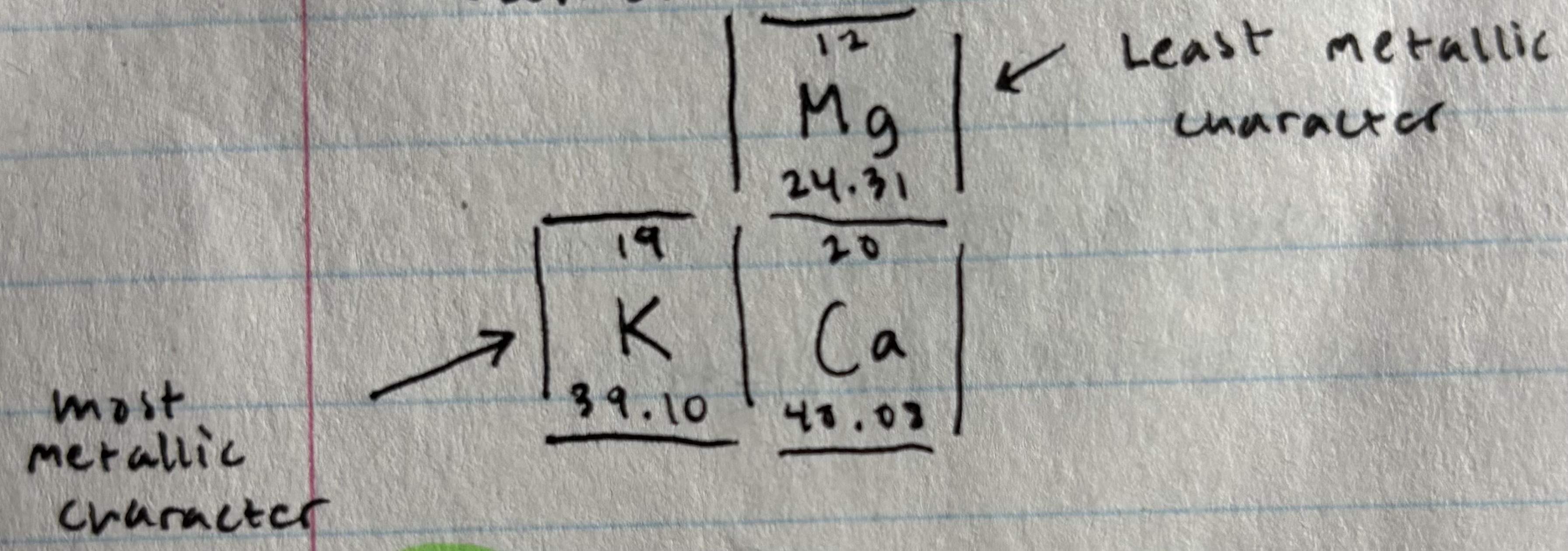
Non-metallic character
Tendency of an element to gain electrons to form negative ions
elements higher up to the right of PT = greatest non-metallic character
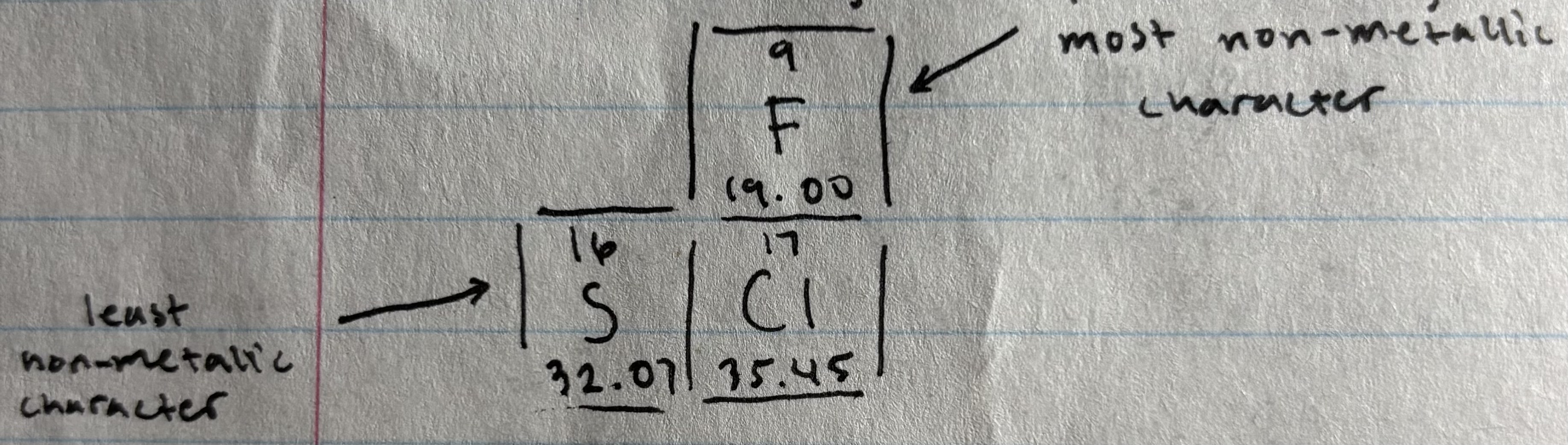
General Trend of Reactivity for Group 1 Metals w/ water
increasing reactivity going down group 1
Going down grp1, elements have lower ionization energies bc of presence of an additional energy level
Its easier for lower ionisation energy to transfer the outer valence electron to water
Halogens
Non metallic elements in group 17 that exist as diatomic molecules
highly reactive with many substances bc tendency to gain electrons
Displacement reaction
Common type of reaction halogens and halide salts readily undergo
AB + C → AC + B
Spectator ion
Aqueous ions that remain unchanged throughout a chemical reaction