EG-335 Thermodynamics and conservation laws
0.0(0)
Card Sorting
1/8
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
Explain the difference between adiabatic process and isentropic process.
An **adiabatic** **process** is defined as a process in which no heat transfer takes place. This does not mean that the temperature is constant, but rather that no heat is transferred into or out from the system.
\
In thermodynamics, an **isentropic** **process** is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter.
\
In thermodynamics, an **isentropic** **process** is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter.
2
New cards
Explain the difference between the model of ideal gas and the perfect gas.
A perfect gas is an ideal gas with constant heat capacities.
\
An ideal gas is one that obeys the ideal gas law (PV = nRT) plus exhibits a heat capacity that does depend on temperature.
\
An ideal gas is one that obeys the ideal gas law (PV = nRT) plus exhibits a heat capacity that does depend on temperature.
3
New cards
Under what conditions is the model of perfect gas is accurate enough?
?
4
New cards
How the ratio of specific heat capacities is related with the number of atoms in the molecule of gas.
One mole of any gas all contain the same number of molecules, NA = 6.022\*10^23
5
New cards
Explain why it is appropriate to deal with specific values for gas flow properties in Gas Dynamics.
Specific values of gas flow properties are essential to determine the value of a property without considering the mass. The property can be easily measured for any amount just by multiplying the specific value with the amount of mass.
6
New cards
Explain the concept of entropy in application to Gas Dynamics
Entropy is a useful quantity to estimate availability of gas system as heat transfer/ friction/shock to/from a gas can change the entropy content of gas. Therefore increased entropy of gas decreases availability of work or vise versa . Entropy generation is caused by shock waves and friction in a gas flow system causing irreversibility/ disorder of gas this disorder can't be reversed .
7
New cards
The change of mass in a control volume equals mass flux through its boundary with the sign minus. Explain why the minus should be used.
We assume a negative mass flux enters a control volume because the outward flow of mass through the volume boundaries is *leaving* the volume. Thus mass over time is negative, resulting in less mass.
8
New cards
Derive the integral form of the mass conservation law for a compressible flow of fluid.
!!!
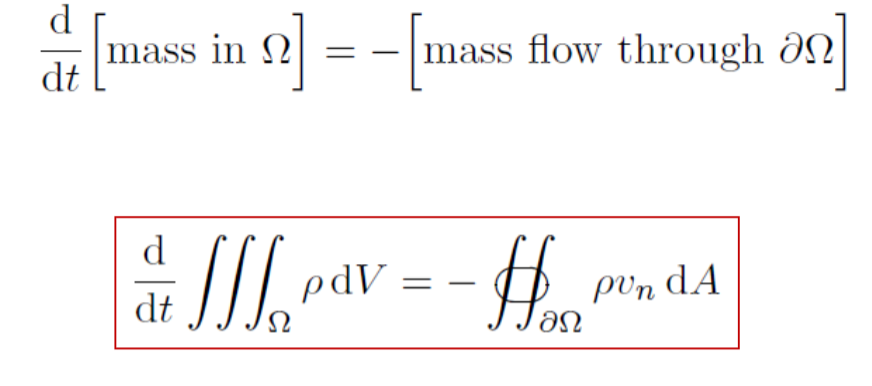
9
New cards
A gas stored in a large reservoir is discharged through a converging nozzle. Considering the process as adiabatic derive the relation between the pressure in the reservoir and the mass of gas stored in the reservoir.
!!!