molecular cell bio exam 3
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
1st law of thermodynamics
energy cannot be created no destroyed, only converted
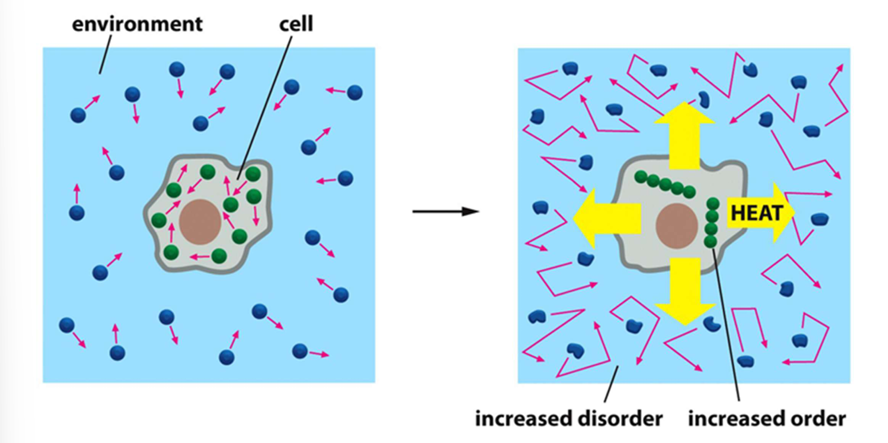
2nd law of thermodynamics
in a closed system, entropy always increases over time —> moving toward disorder
increasing order in the cell generates heat, which dissipates and causes increased disorder outside the cell
3rd law of thermodynamics
as a system approaches absolute zero, all processes cease and the entropy of the system approaches a minimum value
what is energy
capacity to perform work
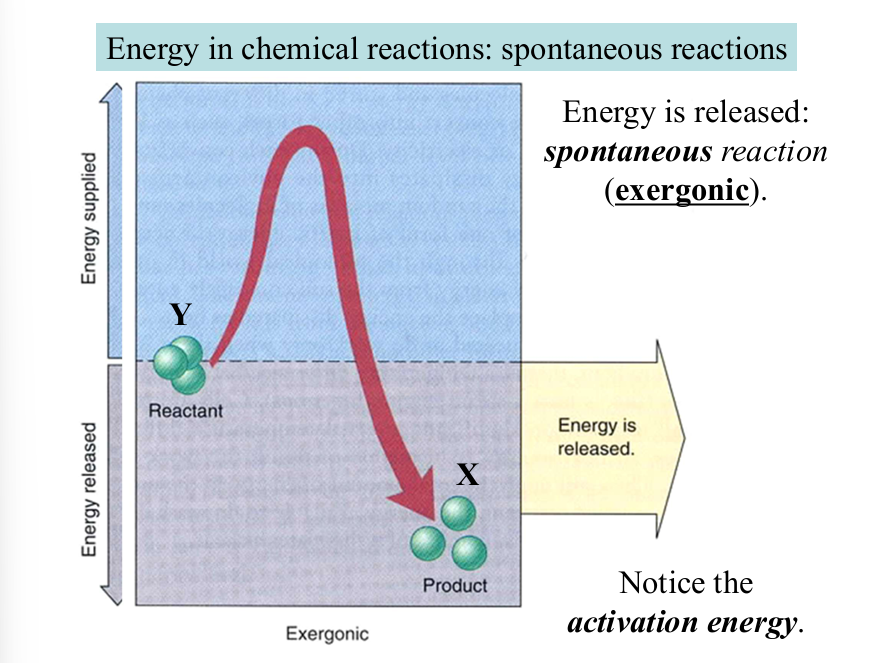
if a reaction results in a negative change in free energy (-changeG), then the reaction increases disorder and is spontaneous
spontaneous
occurs naturally without continuous input of external stimuli
energy is released (exergonic)
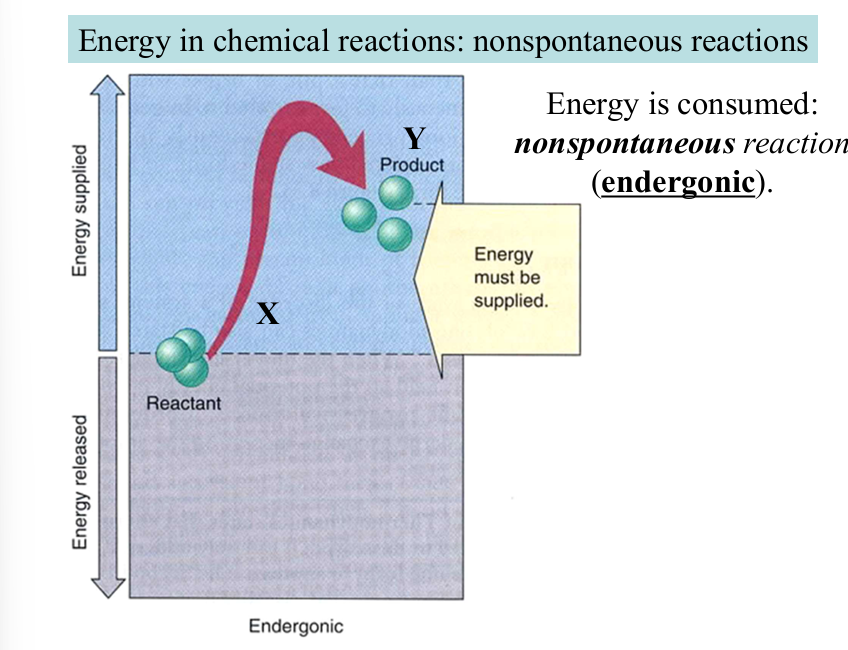
non-spontaneous
requires continuous supply of energy from external sources
energy is consumed (endergonic)
condensation reactions
combines 2 molecules to form single, larger molecule, typically with water expulsion
disaccharide synthesis
dehydration synthesis: non-spontaneous reaction
results in an increase in molecular order (requires energy)
hydrolysis
water breaks a covalent bond, splitting larger molecules into smaller molecules
releases energy (spontaneous)
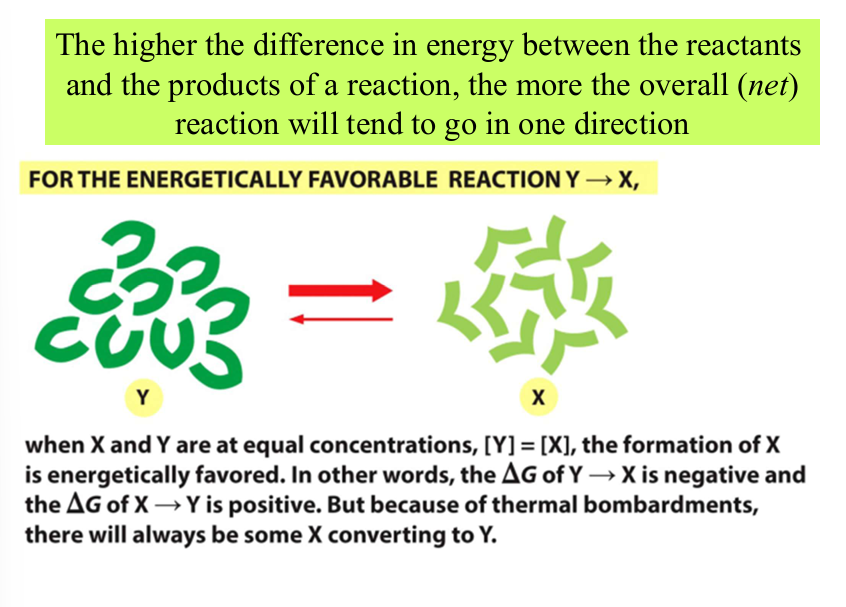
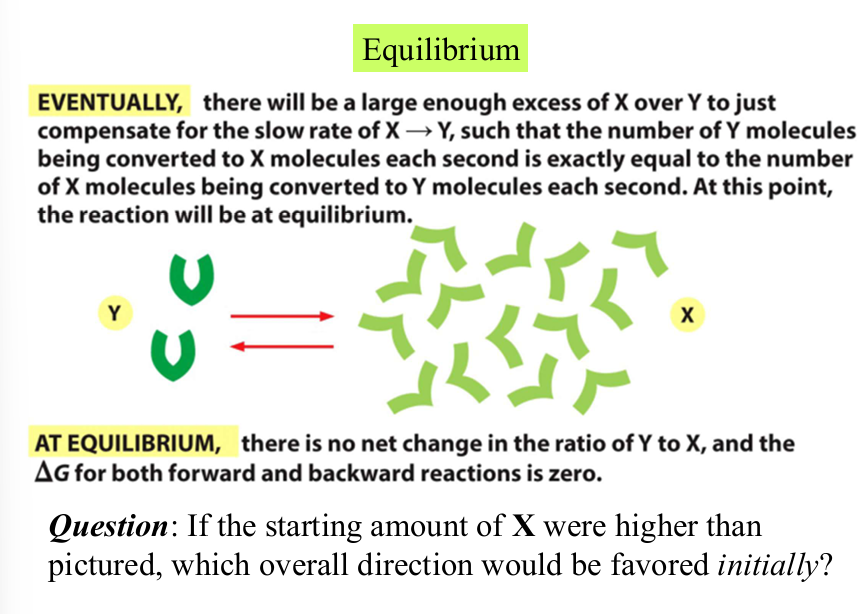
difference in energy vs direction
X has less energy than Y, favored direction will be toward X
Y—>X
equilibrium
state in which no net changes in the amounts of reactants and products occurs
both directions of the reaction occur with the same frequency
as reaction proceeds toward equilibrium, entropy increases
free energy change (ΔG)
reflects the degree to which a reaction creates a more disordered state of the universe
(ΔG) < 0 (negative): spontaneous
(ΔG) > (positive): non-spontaneous
(ΔG) = 0: equilibrium
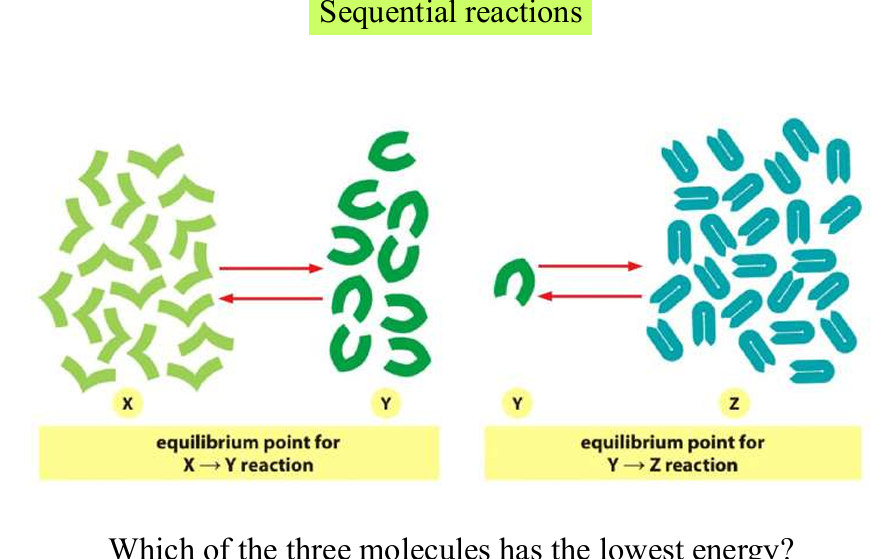
sequential reactions
total free energy change of the overall process is the sum of the energy changes for each individual step
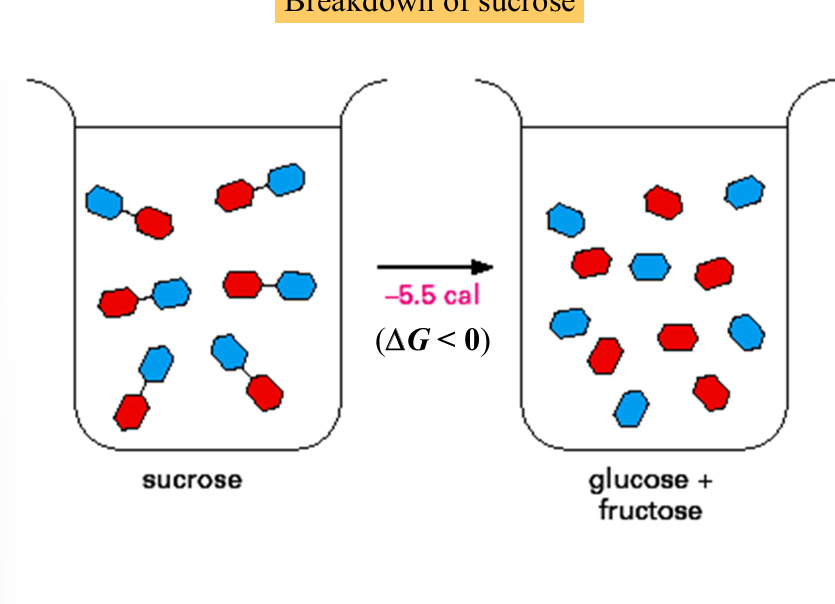
breakdown of sucrose
reaction is exergonic (spontaneous) but must go through a high energy intermediate: the bending of the glycosidic bond and requires activation energy
—> glucose and fructose
enzymes
proteins that act as a biological catalyst by accelerating specific chemical reactions
can lower the activation energy of a reaction and increase rate of reaction
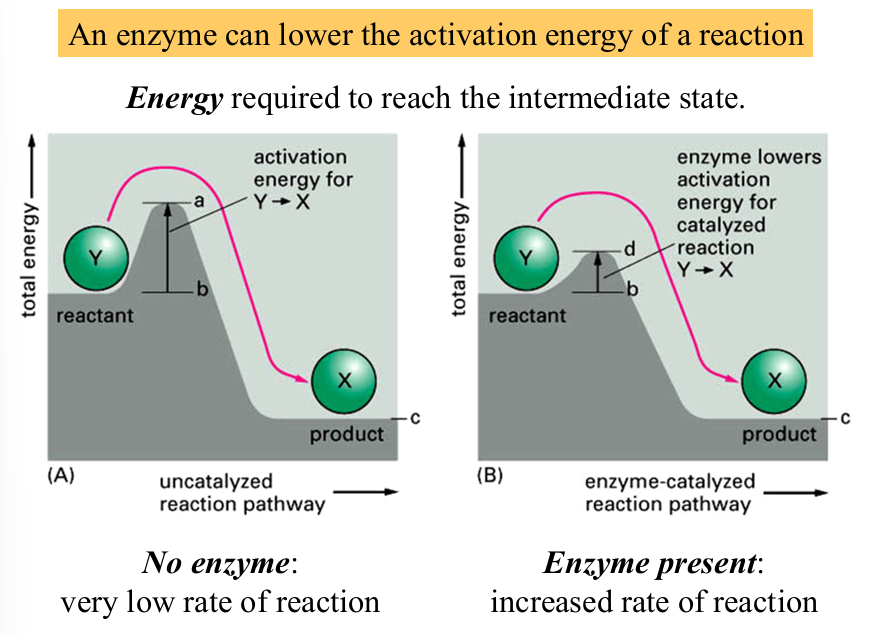
enzyme vs equilibrium point
enzymes speed up rate of a reaction but does not change the equilibrium point
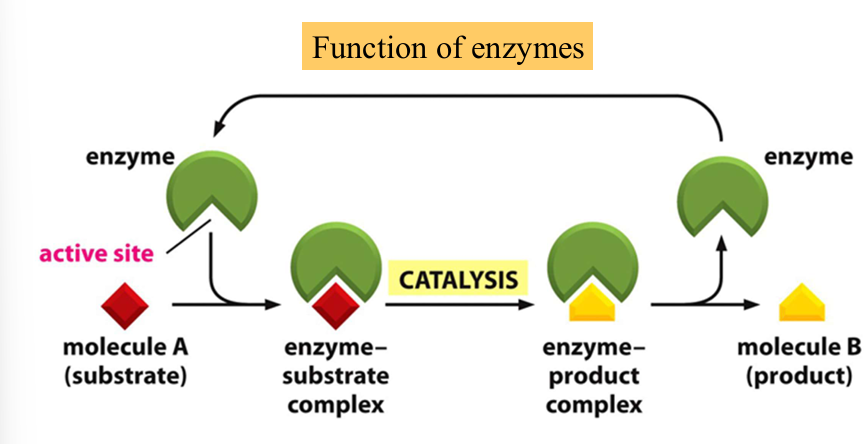
function of enzymes
enzymes catalyze chemical transformations in both directions
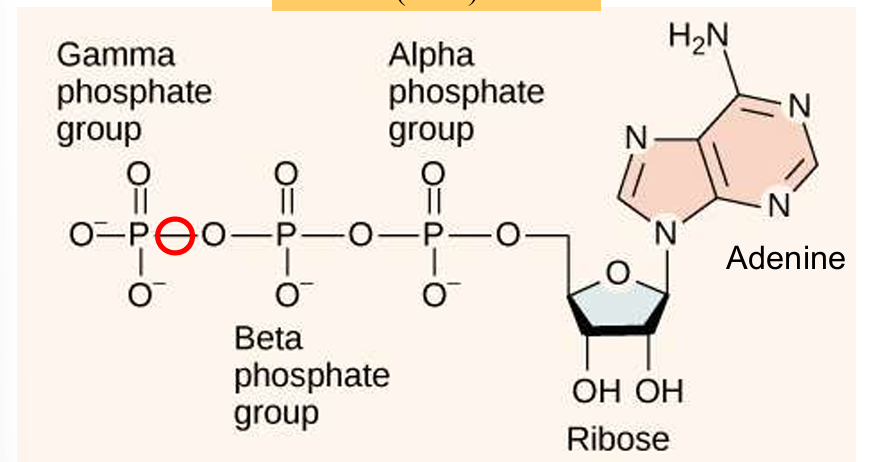
ATP (phosphoanhydride bonds)
high energy bonds between the phosphate groups in ATP are the phosphoanydride bonds
ribonucleotide, energy carrier (source of useful available energy), stores energy
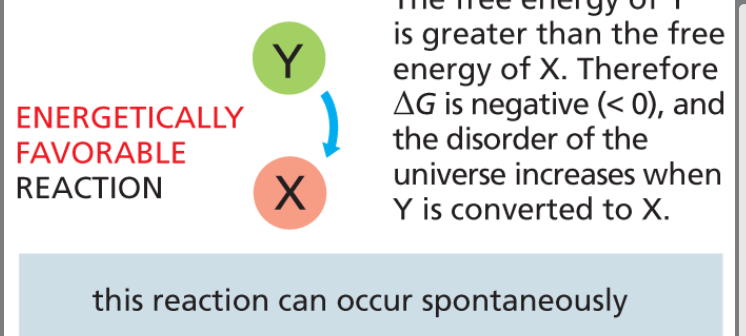
energetically favorable reaction
spontaneous chemical process where the products are more stable and have lower free energy than the reactants -ΔG
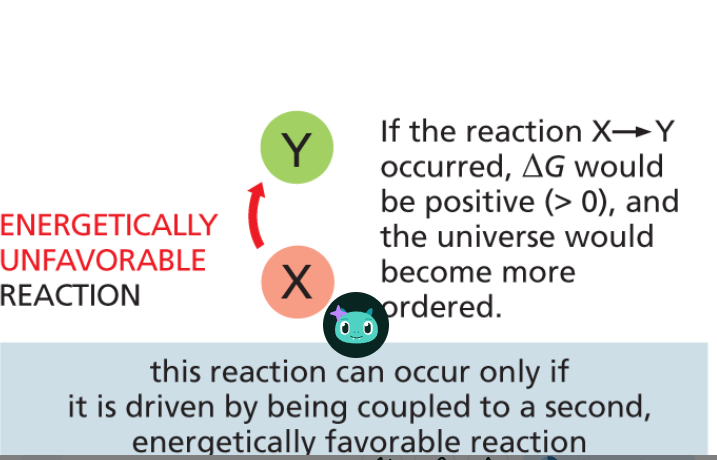
energetically unfavorable reactions
non-spontaneous chemical process resulting in a net increase in free energy +ΔG
energy molecules
ATP is an energy carrier molecule, contains ready to use energy
respiration (energy molecules)
harvesting of the energy stored in macromolecules
oxidation of sugars
catabolic reactions (respiration)
breakdown of food molecules into basic components
their energy is released and transferred to carrier molecules
anabolic reactions (biosynthesis)
use of products of food breakdown as building blocks for the synthesis of necessary macromolecules
requires energy from energy carriers
respiration (breathing)
breakdown of sugars and energy storing molecules
consumes oxygen
releases cos and water
photosynthesis
energy from sunlight is used to build sugars
carbon obtained from co2
water consumed and oxygen released
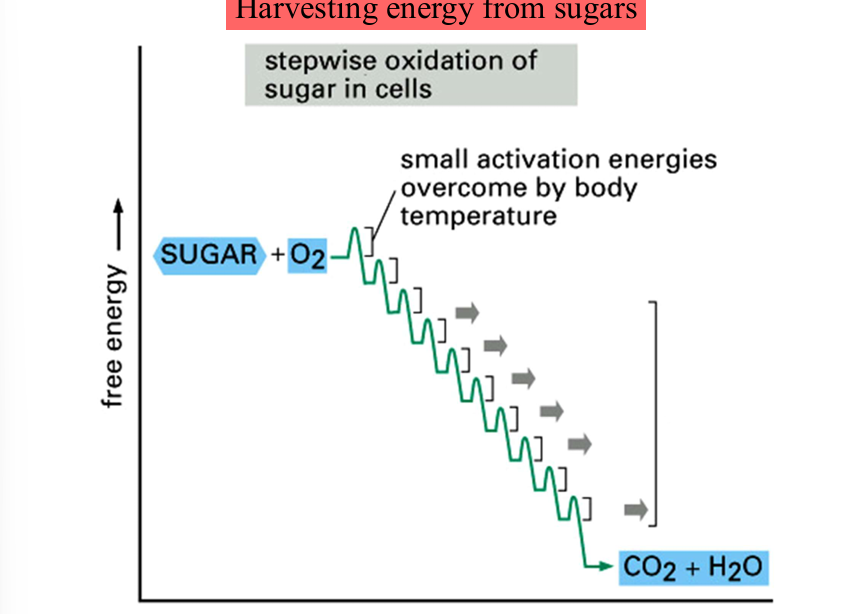
harvesting energy from sugars
slow burning: gradually releasing small pockets of energy
activation energies are lowered by enzymes
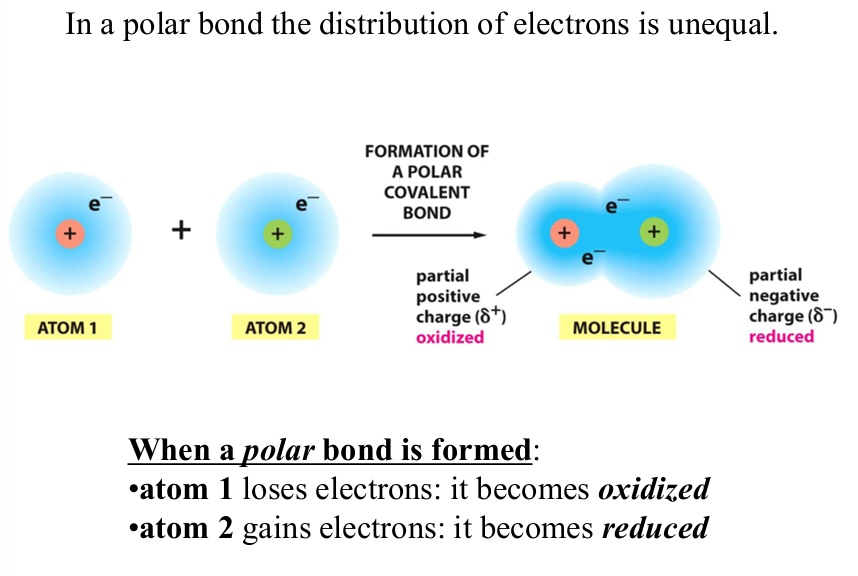
oxidation
reaction where molecules loses electrons
energetically favored
reduction
reaction where molecule gains electrons
energetically unfavorable
glucose has how many carbons?
6
glycolysis phase 1: investment glucose reaction
glucose (6c) —→ glucose 6-phosphate (6c) through
1. phosphorylation with ATP consumed
isomerization to fructose 6-phosphate (6c)
phosphorylation with ATP consumed to fructose 1,6-bisphosphate (6c)
glycolysis phase 2: cleavage
cleavage —→ glyceraldehyde 3-phosphate (3c) + dihydroxyacetone phosphate (DHAP, 3C)
isomerization of DHAP —→ (2) glyceraldehyde 3-phosphate (3c)
glycolysis phase 3: payoff
oxidation with 2NAD+ reduced to 2NADH and an inorganic phosphate (Pi)—→ (2) 1,3-biphosphoglycerate (BPG, 3C)
ATP synthesis by substrate with transfer of 1P from BPG to ADP —→ (2) 3-phosphoglycerate (3c)
isomerization —→ (2) 2-phosphoglycerate (3c)
dehydration with 2H2O removed —→ (2) phosphoenolpyruvate (PEP, 3C)
ATP synthesis by substrate with transfer of 1P from PEP to ADP —→ (2) pyruvate (3c)
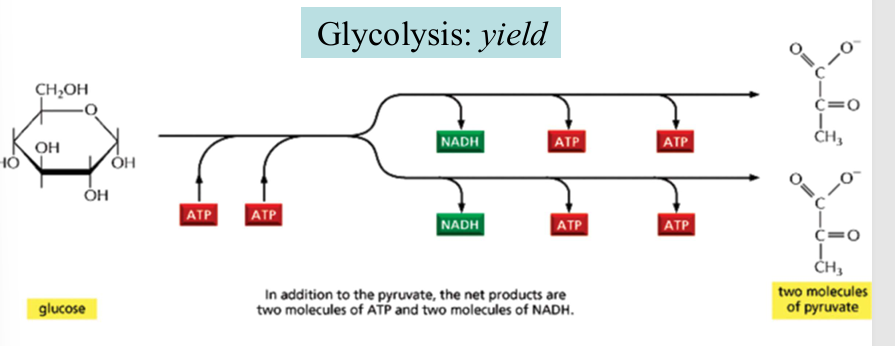
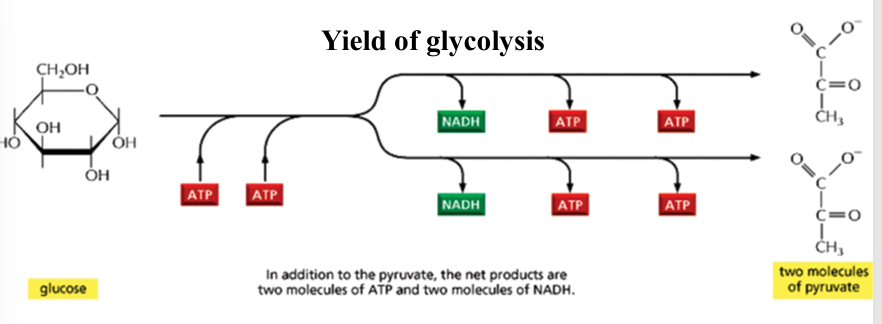
glycolysis: yield
2 ATP consumed at the energy investment phase
4 ATP and 2 NADH produced at the energy generation phases
net yield": 2 ATP + 2 NADH
3 types of muscle fiber
slow muscle fiber: appear dark red b/c of high-density myoglobin and generate their ATP in the mitochondria by oxidative phosphorylation
intermediate muscle fiber: do both
fast muscle fiber: generate ATP by anaerobic respiration
fermentation: anaerobic respiration end products
absence of oxygen
pyruvate is reduced, driven by the oxidation of NADH from glycolysis and getting 2 end products: lactic acid / ethanol + co2
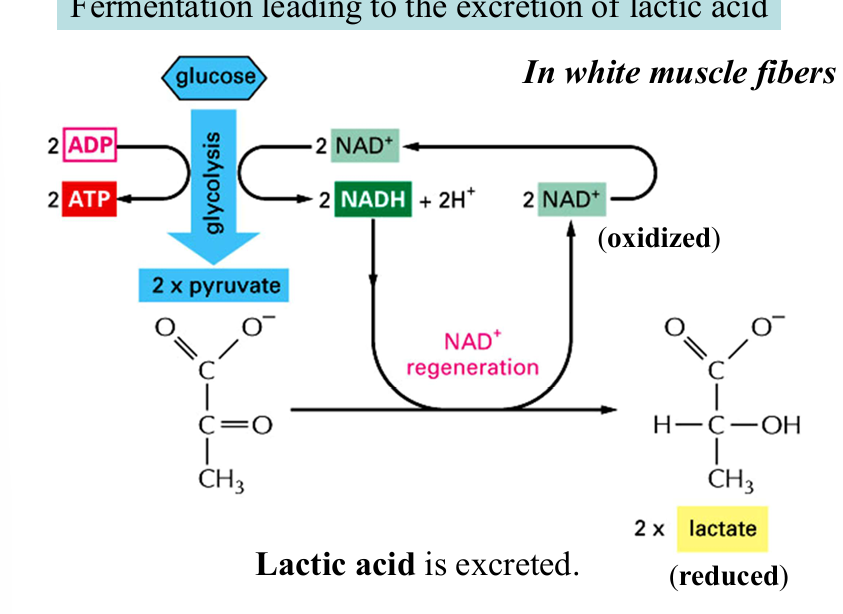
fermentation leading to lactic acid
pyruvate (3c) —→ reduction, NADH oxidized to NAD+ —→ lactic acid (3c)
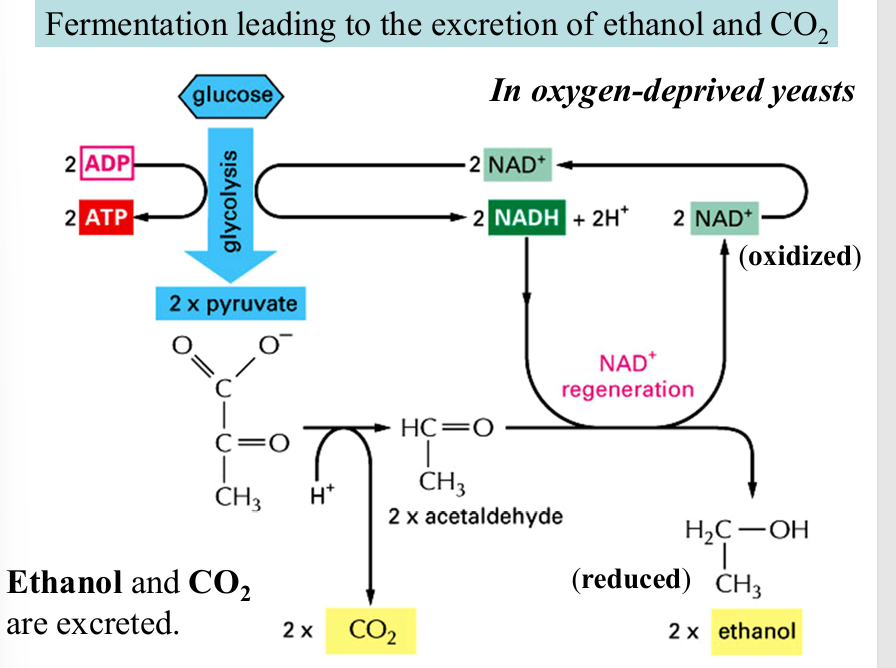
fermentation leading to ethanol and co2
pyruvate (3c) —→ hydrolysis, co2 produced —→ acetaldehyde (2c) —→ reduction, NADH to NAD+ —→ ethanol (2c)
aerobic respiration
pyruvate and NADH go to the mitochondria
pyruvate reacts with the coenzyme A, converted too acetyl coenzyme A, centers the citric acid cycle
NADH powers the oxidative phosphorylation reactions (electron transport chain)
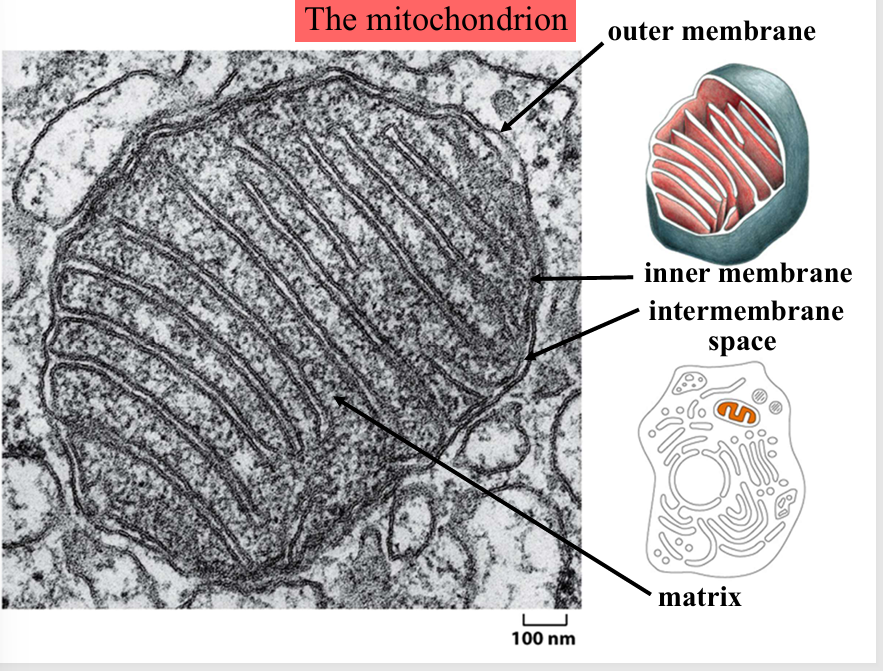
mitochondrion
membrane enclosed organelle that is found in most eukaryotic cells, generates most ATP
the matrix is the site of the oxidation of pyruvate and citrus acid cycle
the inner membrane is the site of electron transport chain
the cristae are infoldings of the inner membrane and contain intermembrane space inside
oxidation of pyruvate
pyruvate: (3C)+CoA
oxidation: CoA-SH + pyruvate —→ acetyl CoA + CO2
CO2 is lost and the intermediate is oxidized
NAD+ is reduced to NADH
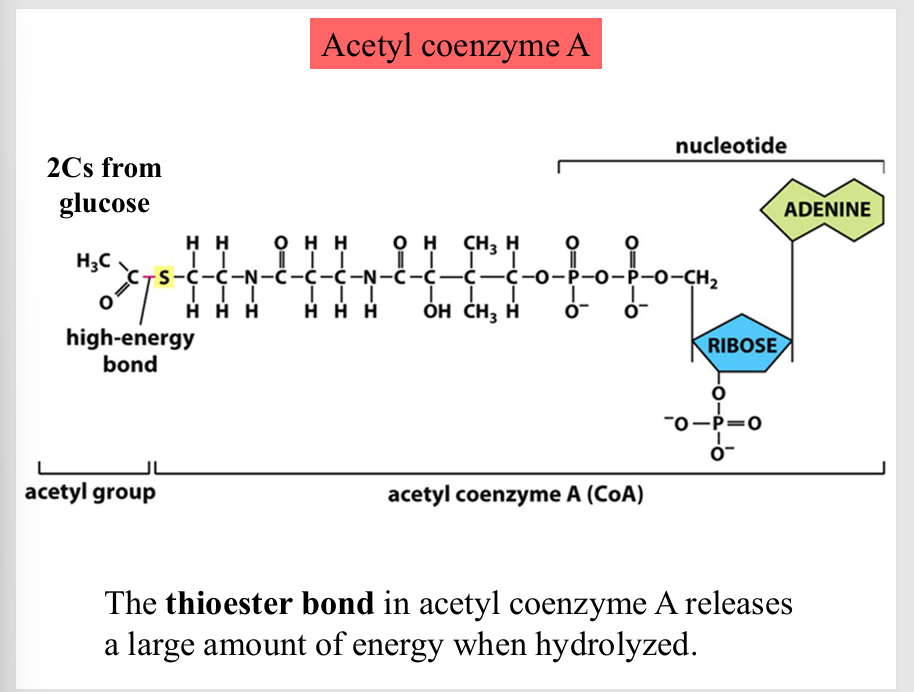
Acetyl coenzyme A
the thioester bond in acetyl coenzyme A releases a large amount of energy when hydrolyzed
citric acid cycle overview (kreb’s cycle/TCA cycle)
2 carbons from acetyl CoA that enter the cycle will not be converted to CO2 in this turn, but will convert in later cycles
the 2 carbons that weren’t converted in the previous cycle will be converted to CO2 now
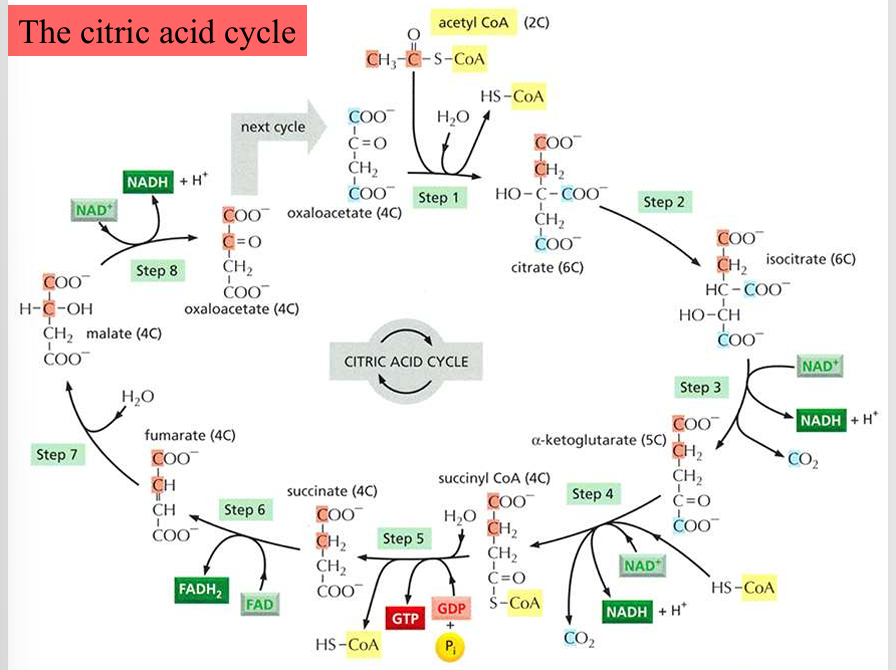
citric acid cycle step 1: condensation
acetyl CoA (2C) + oxaloacetate (4C) —→ citric acid (citrate, 6C)
driven by the hydrolysis that removes CoA-SH
citric acid cycle step 2: isomerization
citric acid (