Farahat Chapter 15 MCQ
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
The rate constant k is dependent on
a. the concentration of the product.
b. the order of the reaction.
c. the temperature.
d. the concentration of the reactant.
e. none of these
c. the temperature.
Which of the following statements is/are true of catalyst?
1. A catalyst is a substance that speeds up a reaction without being consumed itself.
2. A heterogeneous catalyst is one that is present in the same phase (physical state) as the reacting molecules.
3. A homogeneous catalyst is one that is present in a different phase (physical state) from the reacting molecules.
a. 1 only
b. 2 only
c. 3 only
d. 1 and 2
e. 2 and 3
a. 1 only
_____ is defined as the number of species that must collide to produce the reaction indicated by an elementary step.
a. Molality
b. Polarity
c. Delocalization
d. Molecularity
e. Isomerization
d. Molecularity
For which order reaction is the half-life of the reaction independent of the initial concentration of the reactant(s)?
a. zero order
b. first order
c. second order
d. all of these
e. none of these
b. first order
Svante Arrhenius proposed the existence of threshold energy, called the _____, that must be overcome to produce a chemical reaction.
a. lattice energy
b. activation energy
c. kinetic energy
d. ionization energy
e. electrostatic energy
b. activation energy
For which of the following is the half-life directly dependent on the concentration of the reactant?
a. zero-order reaction
b. second-order reaction
c. first-order reaction
d. two of these
e. all of these
d. two of these
If the reaction 2HI → H2 + I2 is second order, which of the following will yield a linear plot?
a. log [HI] vs. time
b. [HI] vs. time
c. ln [HI] vs. time
d. 1/[HI] vs. time
d. 1/[HI] vs. time
24. The half-life is constant.
a. zero order in A
b. second order in A
c. first order in A
d. all of these
e. none of these
c. first order in A
A plot of [A] vs. t is a straight line.
a. zero order in A
b. second order in A
c. first order in A
d. all of these
e. none of these
a. zero order in A
A plot of k vs. 1/T gives a straight line.
a. first order in A
b. zero order in A
c. second order in A
d. all of these
e. none of these
e. none of these
[A] is constant.
a. zero order in A
b. second order in A
c. first order in A
d. all of these
e. none of these
e. none of these
A plot of [A]2 vs. t gives a straight line.
a. zero order in A
b. second order in A
c. first order in A
d. all of these
e. none of these
e. none of these
The rate is constant over time.
a. zero order in A
b. first order in A
c. second order in A
d. all of these
e. none of these
a. zero order in A
The half-life decreases over time.
a. second order in A
b. first order in A
c. zero order in A
d. all of these
e. none of these
c. zero order in A
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
a. second order
b. zero order
c. first order
d. all of these
e. none of these
d. all of these
At a particular temperature, the half-life of a zero-order reaction is 29.0min. How long will it take for the reactant concentration to be depleted by a factor of 8?
a. 87.0min
b. 58.0min
c. 50.8min
d. 232min
e. 203min
c. 50.8min
Rate constants are dependent upon
a. the temperature
b. the probability that a collision will have sufficient energy to cause a reaction
c. the orientation of the collision
d. the frequency of collisions
e. all of the above
f. A and D only
e. all of the above
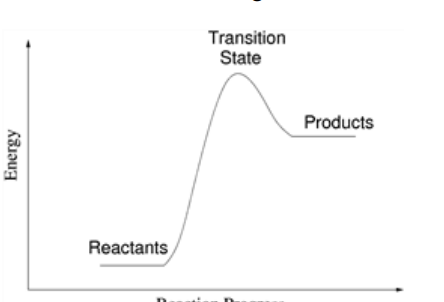
Is this reaction exothermic or endothermic?
Endothermic
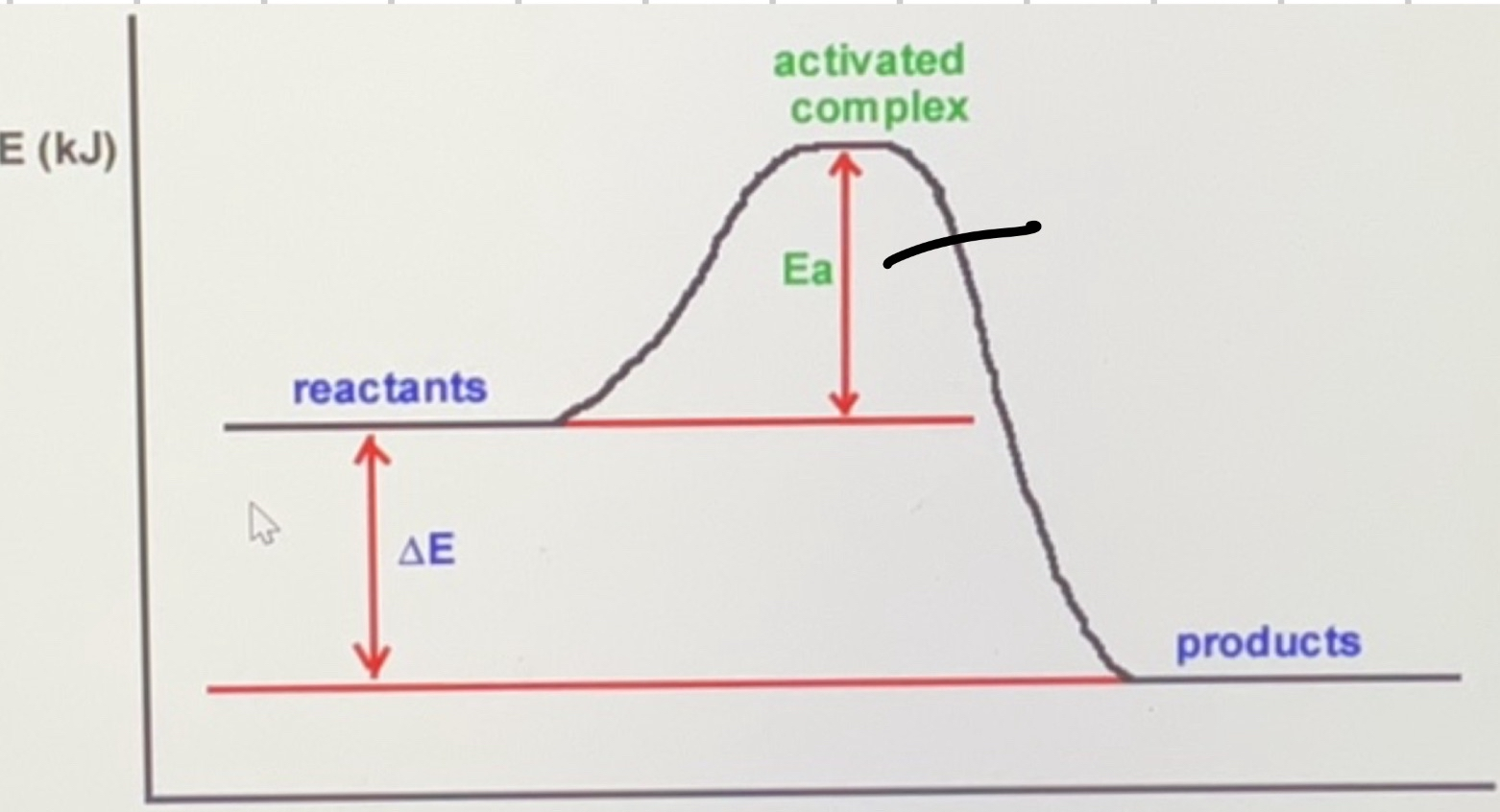
Is this reaction exothermic or endothermic?
Exothermic
Which quantity is greatest?
a. activation energy of the reverse reaction
b. absolute enthalpy of the reaction
c. activation energy of the forward reaction
a. activation energy of the reverse reaction
Which of the following will a catalyst affect?
a. the reaction mechanism
b. the enthalpy of the reaction
c. the activation energy
d. the equilibrium concentrations of products and reactants
e. all of the above
f. A and C only
f. A and C only
Which of the following statement is/are true of rate law?
1. The proportionality constant k, called the rate constant, and n, called the order of the reactant in the rate law, must be determined by experiment.
2. The concentrations of the products of a chemical reaction do not appear in the rate law.
3. The order of a reactant in the rate law can only be a positive integer.
a. 1 only
b. 2 only
c. 3 only
d. 1 and 2
e. 2 and 3
d. 1 and 2
General Rate of Reaction Laws
Rate of consumption = Rate of production = 2(rate of production of other product) (AKA this coefficient needs to go OVER the other coefficient)
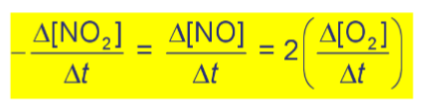
Differential rate law/rate law
Expresses how the rate depends on the concentration of the reactant
Involve concentrations of reactants ONLY
Integrated rate law
Expresses how the concentration of the species in the reaction depends on time
Integrated First-Order Rate Law
The equation expresses the influence of time on the concentration of A (Rate = k[A])
Plot: ln[A] (Y axis) versus t (X axis)
Slope: -k
Half-Life of a First-Order Reaction
Half-life does not depend on concentration (t1/2 depends on k)
A constant time is required to reduce the concentration of the reactant by half and then by half again, and so on, as the reaction proceeds
Second-Order Rate Laws
Plot = 1/[A] versus t
Slope = ONLY ONE THATS POSITIVE (+k)
The equation shows how [A] depends on time (Rate = k[A]2)
Half-Life for a Second-Order Reaction
t1/2 depends on k and [A]0
Each successive half-life is double the preceding one
Zero-Order Rate Laws
Always constant and not dependent on the concentration of the reactant (Rate = k)
The rate will consistently lose so much reactant per unit time.
Slope = -k
Plot = [A] versus t
Half-Life for a Zero-Order Reactions
t1/2 depends on k and [A]0
The second half life will always be half of the first one (i.e. 20 minutes for first → 10 minutes for next)
A → products
Unimolecular
Rate = k[A]
A + A → products (2A → products)
Bimolecular
Rate = k[A]2
A + B → products
Bimolecular
Rate = k[A][B]
A + A + B → products (2A + B → products)
Termolecular
Rate = k[A]2[B]
A + B + C → products
Termolecular
Rate = k[A][B][C]