Chapter 6
1/157
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
158 Terms
Ionic Bonds
occur when the valence electrons of atoms of a metal are transferred to atoms of a nonmetal.
Covalent Bonds
form when atoms of nonmetals share valence electrons.
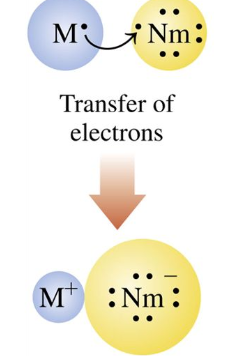
This shows
Ionic Bond
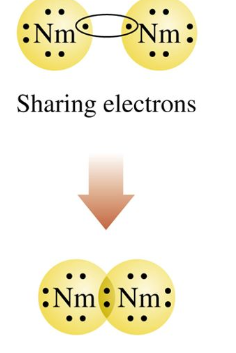
This shows
Covalent Bond
Octet Rule
Each atom acquires an octet of eight valence electrons
An atom that has lost or gained electrons is called an
ion
If metals lose electrons, there would be a
positive charge
When nonmetals gain electrons, there would be a
negative charge

A negative ion is called an
anion
A positive ion is called a
cation
Write the formula and symbol of an ion with 16 protons and 18 electrons.
S2-
Consider the elements calcium and chlorine.
Identify each as a metal or a nonmetal
calcium = metal
chlorine = nonmetal
Consider the elements calcium and chlorine.
State the number of valence electrons for each
Calcium = 2 valence electrons
Chlorine = 7 valence electrons
Consider the elements calcium and chlorine.
State the number of electrons that must be lost or gained for each to acquire an octet.
Calcium = lose two electrons
Chlorine= = gain one electron
Consider the elements calcium and chlorine.
Write the symbol, including its ionic charge, and name of each resulting ion.
Calcium: Ca2+
Chloride: Cl-
The formula of the molecule or compound consists of the
symbols of the atoms found in the molecule
Ionic compounds of made of
positive and negative ions (metals and nonmetals)
Ionic compounds have attractions called
ionic bonds
Ionic compounds have
high melting points
Ionic compounds are
solid at room temperature
In the chemical formula for an ionic compound, the symbols for and subscripts are written in the
lowest whole-number ratio of the atoms or ions
Formula of Ionic Compounds
total positive charge = total negative charge
Write the ionic formula for the compound formed with Ba2+ and Cl- ions
BaCl2
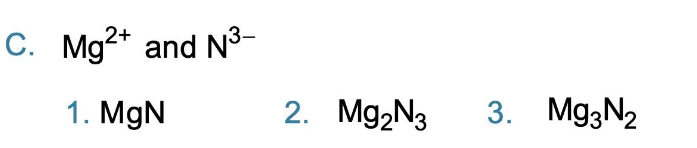
Write the formula for each of the following ionic compounds
3
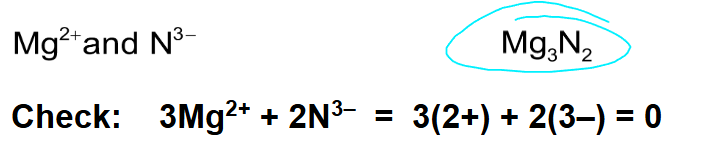
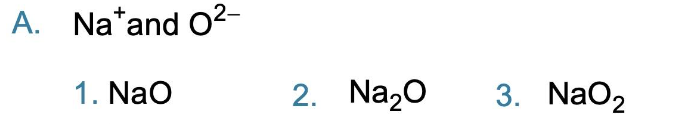
Write the formula for each of the following ionic compounds
2
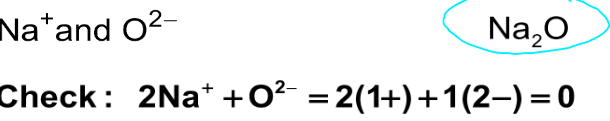

Write the formula for each of the following ionic compounds
1
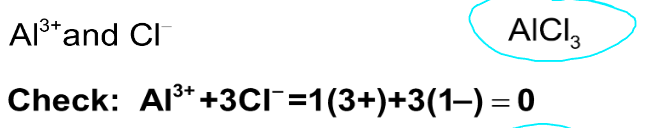
Ionic compounds for between a
metal and nonmental ions
When naming ionic compounds, the name of the metal is written
first
When naming ionic compounds, the name of the nonmetal is written
second
Name the ionic compound K20
Potassium Oxide
Write the names for the following compounds
CaO
Calcium Oxide
Write the names for the following compounds
Al2S3
Aluminum Sulfide
Write the names for the following compounds
MgCl2
Magnesium Chloride
Use the charge on the anion and charge balance to calculate charge on the metal ion
MnF2
MnF2 = manganese (II) fluoride
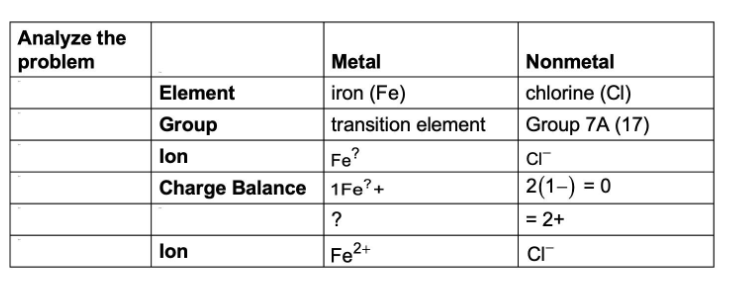
Name the ionic compound FeCl2
Iron (II) chloride
Formula for Variable Change
Name = Metal name (Roman Numeral ion charge) + Nonmetal stem + -ide
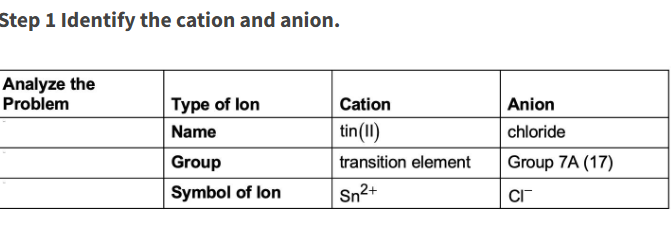
Write the formula for tin (II) chloride
SnCl2
Write chemical formulas for the following compounds
nickel (II) sulfide
nickel (II) sulfide, NiS
Write chemical formulas for the following compounds
Zinc Chloride
zinc chloride, ZnCl2
Write chemical formulas for the following compounds
iron (III) oxide
iron (III) oxide, Fe2O3
Polyatomic Ions
group of atoms with an overall charge
The names of most common polyatomic ions end in
ate
When a related ion has one less O atom, its name ends in
ite
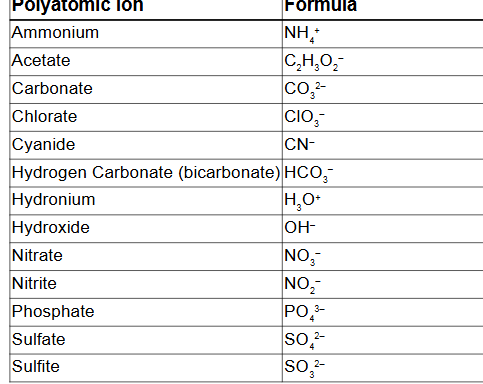
Write the formula for aluminum bicarbonate
Al (HCO3)3
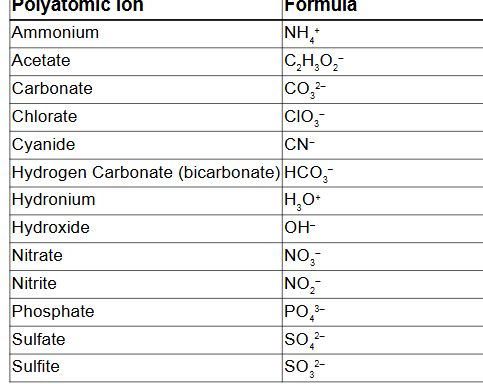
Write the formula for sodium phosphate.
Na3PO4

What is the correct formula for each compound?
3

What is the correct formula for each compound?
2
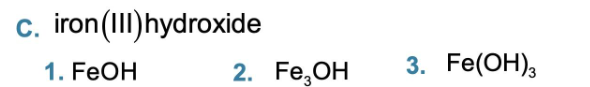
What is the correct formula for each compound?
3
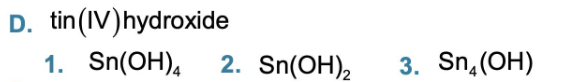
What is the correct formula for each compound?
1
Name the following compounds
Ca(NO3)2
Calcium Nitrate
Name the following compounds
SnSO4
tin(II)sulfate
Name & Formula Ionic Compounds
Main group metals
Name = Metal name + Nonmetal stem + -ide
Formula = Subscripts added to make charges 0
Name & Formula Ionic Compounds
Polytomic Ions
Name = metal name + ion name
Formula = subsripts added to make charges 0 and polyatomic ions in parenthesis
Name & Formula Ionic Compounds
Metals with multiple ions
Name = metal name (roman numeral of ion charge) + nonmetal stem + -ide
Formula = subscripts added to make charges 0
Molecular Compounds
form when atoms of two or more nonmetals share electrons and form a covalent bond
In a covalent bond
valence electrons are shared by nonmetal atoms to achieve stability
A ____ forms when two or more atoms share electrons
molecule
The second nonmetal is named by using the first syllable of its name followed by
ide
The first nonmetal in the formula is named by its
element name
If there is no prefix number, the subscript is assumed to be
one
NO2
nitrogen dioxide
NO3
nitrogen trioxide
Name the molecular compount NBr3
nitrogen tribomide

Choose the correct name for the following molecules
silicon tetrachloride

Choose the correct name for the following molecules
diphosophorus pentoxide

Choose the correct name for the following molecules
Dichlorine heptoxide
Write the name for each molecular compound
Write the name for each molecular compound
CCI4
carbon tetrachloride
Write the name for each molecular compound
CO
Carbon monoxide
Write the name for each molecular compound
N2O
Dinitrogen oxide
Write the name for each molecular compound
PF3
phosphorus trifluoride
____ if the first element in the formula or the name is a ___ or the polyatomic ion NH4+
Ionic; metal
______ if the first metal in the formula is a _____
molecular; nonmetal
Identify each compound as ionic or molecular, and give its correct name
SO3
molecular, sulfar trioxide
Identify each compound as ionic or molecular, and give its correct name
BaCl2
ionic, barium chloride
Identify each compound as ionic or molecular, and give its correct name
(NH4)3PO3
ionic, ammonium phosphite
Identify each compound as ionic or molecular, and give its correct name
Cu2CO3
ionic, copper(I) carbonate
Identify each compound as ionic or molecular, and give its correct name
N2O4
molecular, dinitrogen tetroxide
Covalent Bonds can be represented by drawing the molecule in a
Lewis Structure
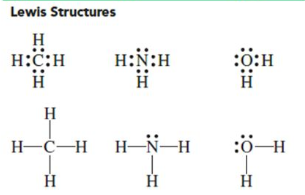
The shared electrons, or bonds, are shown as
two dots or a single line between atoms
Lone pairs
nonbonding pairs of electrons and are placed on the outside as dots
There are ____ naturally occuring diamotic molecules
seven
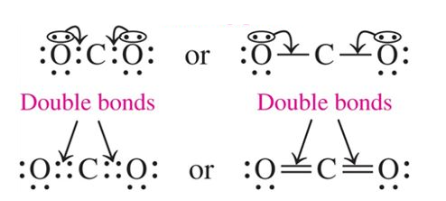
Double Bond
occurs when atoms share two pairs of electrons
Triple bond
occurs when atoms share three pairs of electrons
Draw the Lewis structure for carbon dioxide, CO2 in which the central atom is C
Formaldehyde is a toxic organic molecule with molecular formula CH₂O. Draw the Lewis structure of formaldehyde
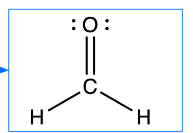
In general, all atoms want an ______ unless they have too few electrons
octet of electrons
Some atoms can have ____________ when they are the central atom
more than eight electrons
In the finished Lewis Dot Structure electrons will come in
pairs
Electronegativity
indicates the attraction of an atom for the shared electrons in a bond.
Electronegativity increases from
left to right and from bottom to top of periodic table
A bond is considered _____, when electrons are shared unequally between atoms
polar
Nonpolar covalent bond
an equal or almost equal sharing of electrons by two bonding atoms
Polar covalent bond becomes more polar as the difference in electronegativity
increases
Nonpolar and polar covalent bonds occurs between
nonmetals
Polar covalent bond
unequal sharing of electrons
Dipole
separation of charges in a polar bond
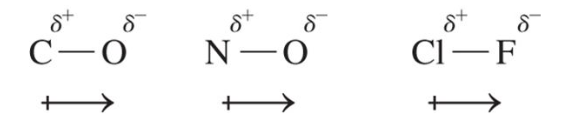
This shows
dipole
Ionic bond
result of electron transfer with a large electronegativity difference
Ionic bonds occurs between
metals and nonmetal ions