Chemistry Test :Atomic Structure and Electron Configuration
1/9
Earn XP
Description and Tags
Flashcards for Chemistry Test: Atomic Structure and Electron Configuration
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Atomic Number
The number of protons in an elemen. Also, in a neutral atom, the number of protons equals the number of electrons.
Mass Number
It can be used to determine the number of neutrons by subtracting the number of protons from the mass number.
Electron Configuration Sequence (up to 4p)
1s² 2s² 2p^6 3s² 3p^6 4s² 3d^10 4p^6
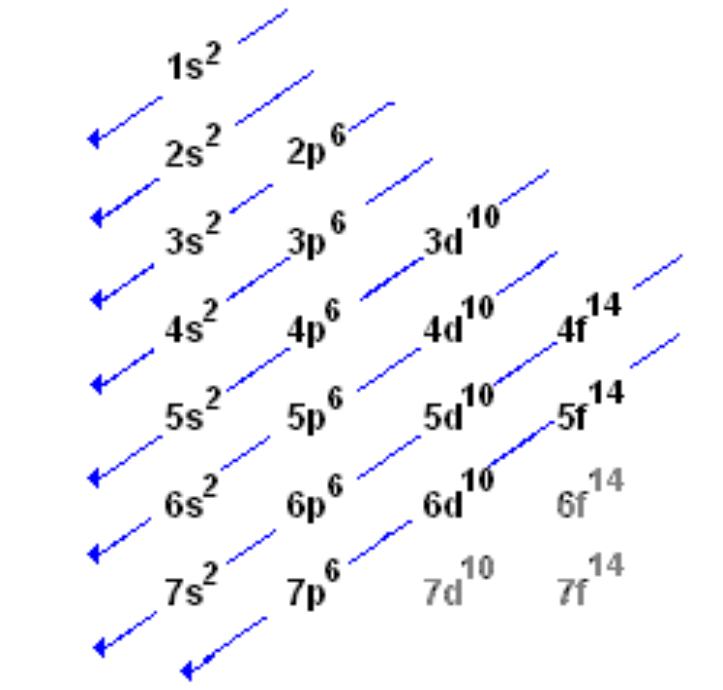
Period Number
Represents the energy level (shell) of an element, indicating the number of electron shells.
Group Number
Indicates the number of valence electrons in the outermost shell of an element.
isotope
atoms w the same number of protons but diff numbers of neutrons
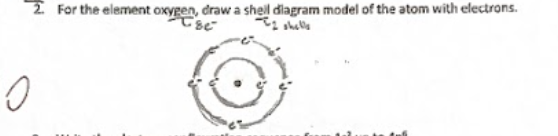
ex shell diagram
A visual representation showing the arrangement of electrons in different energy levels around an atom's nucleus. It helps illustrate how electrons are distributed among the shells.
electrons in an atom = atom number (ex oxygen has 8)
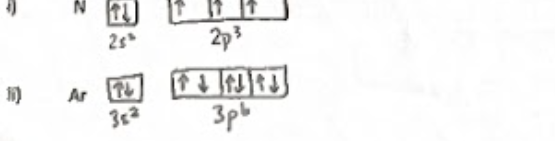
orbital diagram
based on electron configurationthat visually represents the distribution of electrons in an atom's orbitals, showing the occupancy of each orbital with arrows for each electron.
Atomic mass formula
Two isotopes of [element] has a mass of X amu and Y% abundance while the 2nd isotope has a mass of Z amu and W% abundance. What is the atomic mass of [element]?
(x)(y%/100)+(z)(w%/100)
% abundance
two isotopes. 1st: x amu and y% abundance. Other isotope has z amu. What is the % abundance?
subtract 100-first isotope amu (logic bru)