Transition Metals
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
How do transition metal characteristics arise?
The characteristics of elements titanium to copper arise from an incomplete d sub level in atoms or ions
What are the four characteristics of a transition metal?
Complex formation
Formation of coloured ions
Variable oxidation state
Catalytic activity
Why are scandium and zinc not a transition metal?
They don’t have variable oxidation states, and have a complete d orbital and so doesn’t meat the criteria of incomplete d orbital
What is a complex?
A central metal ion surrounded by ligands
What is a ligand?
An atom, ion or molecule which can donate an electron pair
What is co-ordinate bonding?
When the shared pair of electrons in the covalent bond comes from only one of the bonding atoms
What is co-ordination number?
The number of co-ordinate bonds to a central metal atom or ion
What are the different types of ligands and give examples?
Monodentate- form one coordinate bond per ligand e.g. water, NH3 and Cl-
Bidentate- two atoms with lone pairs which can form two coordinate bonds per ligand e.g. 1,2-diaminoethane and ethanedivoate ion
Multindentate e.g. EDTA4-
What is a ligand substitution reaction?
One in which one ligand is coordinately bonded to a metal atom/ion and is replaced by another ligand
What is similar about the ligand NH3 and H2O and their reactions?
Similar in size and uncharged
So exchange of these ligands occurs without change of the co-ordination number e.g. with copper and cobalt
[Co(H2O)6]2+(aq) + 6NH3 (aq) [Co(NH3)6]2+(aq) + 6H2O (l)
In the case of copper the substitution may be incomplete:
e.g. [Cu(H2O)6]2+(aq) + 4NH3 (aq) to [Cu(NH3)4(H2O)2]2+ (aq) + 4H2O (l)
How does the reaction with chloride ions differ?
The chloride ion ligand is larger than the uncharged water and ammonia, so therefore ligand exchange can involve a change of coordination number
e.g. addition of concentrates HCl to aqueous ions of copper and cobalt leads to a change in coordination number from 6 to 4
[Cu(H2O)6]2+ + 4Cl- to [CuCl4]2- + 6H2O [Co(H2O)6]2+ + 4Cl- to [CoCl4]2- + 6H2O
How does this differ when the solid metal chloride is dissolved in water?
If dissolved in water, it forms the aqueous hexaaqua (metal) complex, not the chloride complex
e.g. [Cu(H2O)6]2+ not [CuCl4]2-
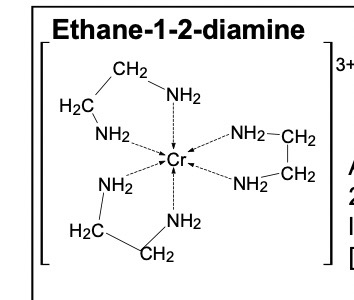
Draw the structure of the bidentate ligand 1,2- diaminoethane
There are 3 bidentate ligands in this complex, each bonding in twice to the metal ion. It has a coordination number of 6
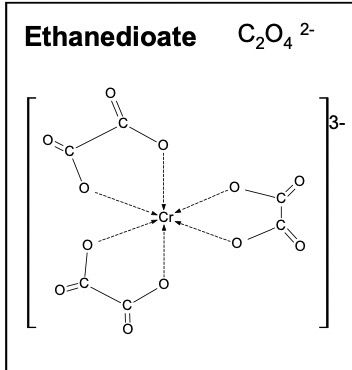
Draw the structure of the bidentate ligand ethandioate ion
C2O42-
Octahedral shape with 90 degree angles
What are the equations to show formation of bidentate ligands?
[Cu(H2O)6]2+ + 3NH2CH2CH2NH2 to [Cu(NH2CH2CH2NH2)3]2+ + 6H2O
[Cu(H2O)6]2+ + 3C2O42-to [Cu(C2O4)3]4- + 6H2O
Draw the structure of the multidentate ligand EDTA4-
Forms six coordinate bonds per ligand
[Cu(H2O)6]2+ + EDTA4-to [Cu(EDTA)]2- + 6H2O
![<ul><li><p>Forms six coordinate bonds per ligand </p></li><li><p><span>[Cu(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup> + EDTA<sup>4-</sup>to [Cu(EDTA)]<sup>2-</sup> + 6H<sub>2</sub>O</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/99eb770b-f5e2-4d7a-9cef-cbb589c410b8.jpg)
What is an example of an iron complex with a multi dentate ligand?
Haem
Each Fe2+ containing harm complex can accept a pair of electrons and form a coordinate bond from an oxygen molecule. This is how oxygen is transported around the blood
Why does this relate to how carbon monoxide is toxic?
Carbon monoxide also contains a lone pair of electrons on the oxygen atom, and this can form a stable complex with haemoglobin
When incomplete combustion of fuels occur and CO is produced, as when it replaces the oxygen it prevents oxygen being carried in the blood
How can a complex be made more stable?
This substitution of a monodentate ligand with a bi/multidentate ligand leads to a more stable complex. This is called the chelate effect
How can we explain the chelate effect?
Positive entropy change, as there are more moles of products than reactants
When there are more moles of products than reactants, there is an increase in entropy which creates more disorder
If there are a similar number bonds in both complexes, the enthalpy change will be small and change in entropy is positive, so Gibbs will be negative, so a stable complex is formed
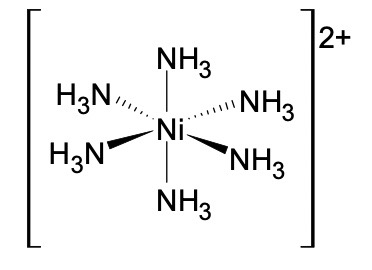
What is the shape of a complex ion with six coordinate bonds?
Octahedral
TM ions commonly form octahedral complexes with small ligands like water and ammonia
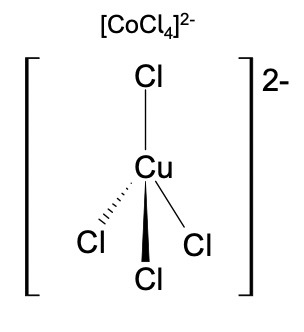
What is the shape of a complex ion with four coordinate bonds?
Tetrahedral
forms with larger ligands like chloride ions
What shape do platinum and nickel end up forming?
Square planar e.g. cisplatin
No overall charge
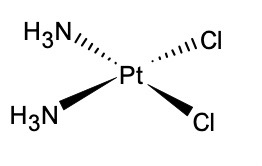
What is the shape of a complex ion with two coordinate bonds?
Linear
[Ag(NH3)2]+ as used as Tollen’s reagent
![<p>Linear </p><p><span>[Ag(NH<sub>3</sub>)<sub>2</sub>]<sup>+ </sup>as used as Tollen’s reagent</span></p>](https://knowt-user-attachments.s3.amazonaws.com/bf9c33c1-69c9-4542-9ae1-76a907735413.jpg)
How is stererisomerism shown in these shapes?
cis-trans isomerism in square planar complexes
only the cis isomer of the cisplatin drug shows any anti-cancer activity
cis= z trans= e
Cis-trans isomerism in octahedral complexes
Optical isomerism in octahedral complexes

What three things do colour changes arise from?
Oxidation state
Co-ordination number
Ligand
How do colour arise?
In these coloured compounds and complexes, the d sub shell must be partially filled
The d sub shell is split into two distinct sets of orbitals that have a difference in energy between them
Energy in the visible region causes d electron transition and electrons become excited, so are promoted to the higher energy orbitals (excited state) from the ground state
A portion of visible light is absorbed to promote the d electrons to higher energy levels; the light that is not absorbed in transmitted to give the substance colour
What is the equation linking colour, wavelength and frequency of light absorbed?
energy difference between orbitals= frequency of light absorbed x Planck’s constant
or
energy difference between orbitals= (planks constant x speed of light) / wavelength of light absorbed
How do we change colours?
Changing a ligand or changing coordination number will alter the energy split between the d orbitals, change the energy difference and hence the frequency of light absorbed and transmitted
Why is absorption of visible light used in spectrometry?
To determine the concentration of coloured ions
If visible light of increasing frequency is passed through a sample of coloured complex ion, some of the light is absorbed
The amount of light absorbed is proportional to the concentration of the absorbing species
Complexes which have only a pale colour and do not absorb light strongly have a suitable ligand added to intensify the colour
Spectrometers contain a coloured filter, which is chosen to allow the wavelengths of light through that would most strongly be absorbed by the coloured solution
How is a colorimeter used to determine conc of an coloured ions in a solution?
Add an appropriate ligand to intensify colour
Make up solutions of known concentration
Measure absorption or transmission using colorimeter
Plot graph of absorption vs concentration
Measure absorption of unknown and compare
How is vanadium reduced into its successive oxidation states?
Addition of zinc in acidic solution- strong reducing agent
What are vanadium’s 4 main oxidation states and their colour?
VO2+ Oxidation state +5 ( a yellow solution)
VO2+ Oxidation state +4 (a blue solution)
V3+ Oxidation state +3 (a green solution)
V2+ Oxidation state +2 (a violet solution)
What is the redox potential influenced by?
The redox potential for a transition metal ion changing from a higher to a lower oxidation state is influenced by pH and by the ligand.
[Ag(NH3)2]+ is used in Tollens’ reagent to distinguish between aldehydes and ketones. Aldehydes reduce the silver in the Tollens’ reagent to silver.
Red 1⁄2 eq: [Ag(NH3)2]+ + e- Ag +2NH3
Ox 1⁄2 eq: CH3CHO + H2OCH3CO2H + 2H+ + 2e-
What is a catalyst?
A substance that increases rate of reaction but is not used up itself, by providing an alternative pathway with a lower activation energy. It speeds up rate at which an equilibrium is reached by speeding up the forwards and backwards reaction by the same amount
What are the two types of catalyst?
Homogeneous and heterogeneous
What is a homogeneous catalyst?
In the same phase as the reactants
How do homogeneous catalysts work?
They proceed via an intermediate species formed from a reactant and the catalyst, which reacts further and regenerates the catalyst
What are examples of homogeneous catalysts?
Fe2+ and Fe3+ can catalyse the reaction between iodide ions and peroxodisulfate ions.
S2O82- + 2Fe2+ to 2SO42- + 2Fe3+ and then:
2Fe3+ + 2I- to I2 + 2Fe2+
Overall equation is:
S2O82–(aq) + 2I– (aq) ==> 2SO42–(aq) + I2(aq)
At first, without the catalyst, the reaction is slow because S2O82–and I– are both negative ions and repel, so higher activation energy. The iron (II) is attracted to the peroxodisulfate so lowers the activation anergy.
Iron (II) is the catalyst because the oxidation state has changed/ variable
Conc hydrogen sulphate can be used to catalyse esterification between carboxylic acid and alcohol
Depletion of ozone: chlorine gas is the catalyst and is in the same phase as ozone gas when ut increases the destruction of the ozone layer
What is a heterogeneous catalyst?
Different phase from reactant (e.g. solid and gas). Catalyst is usually solid and reaction takes place on surface with gases and solutions which lowers activation energy to speed up reaction
What are examples of heterogeneous catalysts?
Catalytic cracking: catalyst is zeolite/ aluminium
Catalytic converters: made of platinum, palladium, and rhodium
The Haber process: catalyst is iron, used to increased RoR between nitrogen and hydrogen to produce ammonia
Contact process: catalyst is vanadium oxide (V2O5), used to increase RoR between SO2 and O2
Why are heterogeneous catalysts often coated on an inert support medium?
Thin coating can be used to maximise surface area, as metals are expensive so minimises costs
How do heterogeneous catalysts works?
Reactant molecules are adsorbed onto active sites on the surface of the catalyst
The bonds in the reactant molecules are weakened
The reactant molecule are held in a more favourable conformation for reaction
The product molecules are desorbed from the surface of the catalyst
How can heterogeneous catalysts become poisoned?
May be poisoned by impurities in the reaction mixture
Catalyst is poisoned as the active sites on the surface of catalyst become blocked and therefore inactive, and consequently have a reduced efficiency, which has cost implications
What things would make a heterogeneous catalyst more/ less efficient?
Adsorb the reactant for so long the reactants stay in the active sites to react
Desorb the reactants quickly enough that the active sites free up and can adsorb more reactants
Poisoning of catalyst and so blocking of active sites makes less efficient
What is autocatalysis?
Creates the catalyst itself in the reaction
What are the equations and explanation for how V2O5 acts as catalyst in the contact process?
SO2 + V2O5 to SO3 + V2O4 (reduction)
V2O4 + 0.5O2 to V2O5 (oxidation)
Overall equation: 2 SO₂(g) + O₂(g) → 2 SO₃(g)
Variable oxidation states throughout these reactions
How does Mn2+ autocatalyse the reaction between C2O42- and MnO4-?
Oxidation of ethanedioic acid:
C2O42- to 2CO2 + 2e-
Reduction of MnO4- to Mn2+
MnO4- + 8H+ + 5e- to Mn2+ + 4H2O
Overall equation
5C2O42- + MnO4- + 16H+ to Mn2+ + 8H2O + 10CO2Mn2+ : autocatalyst
Catalyses reaction by reacting with manganate to form manganese:
2MnO4- +4Mn2+ + 8H+ to 5Mn3+ + 4H2O
And then the Mn3+ reacts with ethanedioic:
2Mn3+ + C2O42- to 2CO2 + 2Mn2+
Overall equation:
C2O42- + MnO4- + 16H+ to 2 Mn2++ 8H2O + 10CO2
Outcome is the same but has been sped up by manganese, as it is a positive catalyst in the mix with negative ions so no repulsion to overcome
Why does Zn2+ not catalyse reactions?
Only one oxidation state