BACT 211: ACID FAST STAINING
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Acid-fast Stains
Differential staining useful in identification of acid-fast bacilli (AFB)
Higher affinity for primary stain (can’t uptake counterstain)
Resists decolorizer
Aside from identification of AFB, what is the other use for acid-fast stains?
Preliminary diagnosis of Tuberculosis
N-glycolylmuramic acid
Found in mycobacterial cell wall
N-glycolylmuramic acid.
Also known as “Mycolic acid.”
N-acetylmuramic acid
Found in gram (+) bacterial cell wall
Pink/Red
This
Other Acid-fast Microorganisms
Tsukamurella
Gordonia
Nocardia
Corynebacterium
Rhodococcus
Parasites that are Partially Acid-fast
Cryptosporidium
Isospora
3% sulfuric acid
Alternative decolorizer for partially acid-fast parasites
Smear Positive
Most common method to confirm presence of TB
DOH-based Smear
2 samples (early morning → random)
w/n 1 day
½ is positive
Book-based Smear
3 samples (all early morning)
3 consecutive days
2/3 is positive
Culture Positive
Löwenstein-Jensen Medium
M. tuberculosis grows w/n 8 weeks or 2 months
Cauliflower appearance
Malachite green for sample decontamination
Petragnani
High conc. of Malachite green
Used for heavily contaminated samples
Rapid Diagnostic
Molecular approach
PCR-based
Useful for identifying if patient is resistant to anti-TB meds
Purified Protein Derivative (PPD) Test
“Mantoux test”
For identification of latent TB, 48 hrs (3rd type)
Uses ammonium sulfate (for heat killing)
Skin reaction = probably positive
Bacillus Calmette-Guerin
Vaccine that creates false positive result in PPD test
For M. bovis
Sputum
Most common sample for acid-fast staining
Gastric aspirate
Sample used for infants
Cerebrospinal Fluid
For identification of Tubercular meningitis (2nd type)
Web-like pellicle appearance after refrigeration
3P’s Sample
Pleural (lungs)
Pericardial (heart)
Peritoneal (abdomen)
Antibiotics used for TB
Rifampin
Isoniazid
Pyrazinamide
Ethambutol
Streptomycin
5-10 mL
Min-max volume of sputum sample required
More than 25 epithelial cells per low power field
This sputum sample is rejected because it has higher contents of saliva
Low Power Objective (LPO)
An objective lens used for sample viability
Oil Immersion Objective (OIO)
An objective lens used for reading smear preparation
Smear Preparation
Coiling method (small circular motion)
Size: 2 × 3 cm
Thumb-shaped
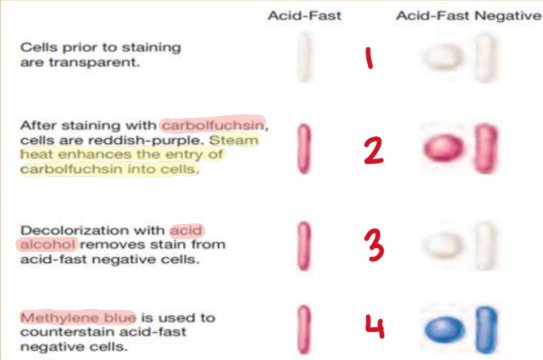
Ziehl-Neelsen Method
“Hot method”
Primary stain w/ Carbol Fuchsin
Heat until boiling
Cool down for 5 mins
Wash off excess w/ water
Decolorize w/ acid alcohol (HCL) for 30 secs
Counterstain w/ Methylene Blue or Malachite Green for 30 secs
Wash off excess w/ water
Kinyoun’s Method
“Cold method”
Primary stai: more lipid soluble & concentrated Carbol Fuchsin
Uses Tergitol as mordant instead of heat
high phenol conc.
Same procedure w/ hot method but is preferred
Baumgarten’s Method
Mixture of 10 parts (95% alcohol) and 1 part (nitric acid)
Differentiation of M. tuberculosis (stained blue) vs. M. leprae (stained red)
Gabbet’s Method
Uses Gabbet’s Methylene Blue as both decolorizer and counterstain
Pappenheim’s Method
Uses Pappenheim’s differentiating stain
Differentiates M. tuberculosis (stained red)vs. M. lacticola or M. smegmatis (both stained blue)
Modified-Acid Fast Stain
Stain used for partially acid-fast parasites
uses 3% sulfuric acid as decolorizer
Fite-Faraco’s
Identification of M. leprae
Uses “Hematoxylin” as counterstain instead of Methylene Blue
Auramine-Rhodamine
“Truant’s Method”
Uses fluorescent dye and fluorescent microscope
AFB = yellow appearance under black background
Spengler’s Method
Used by colorblind individuals
AFB = black appearance
Smear Result: 0
No AFB seen
Smear Result: +/-
1-2 per 300 fields
Smear Result: 1+
1-9 per 100 fields
Smear Result: 2+
1-9 per 10 fields
Smear Result: 3+
1-9 per field
Smear Result: 4+
Greater than 9 per field