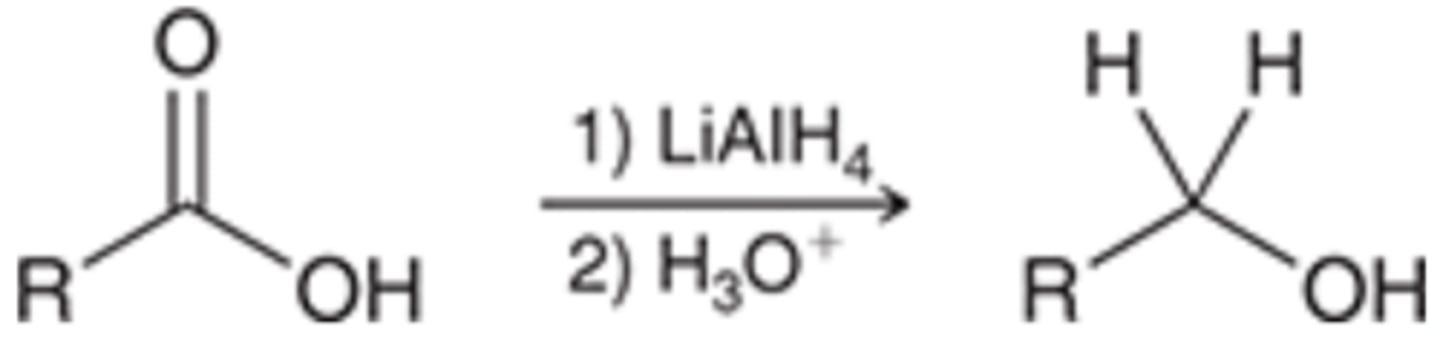Chapter 19: Carboxylic Acids
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
Reactivity of Carboxylic Acids
- carboxylic acids have high boiling points due to the stronger intermolecular forces (H-bonding)
- a strong base (like NaOH) is required to completely deprotonate a carboxylic acid
- carboxylic acids are more acidic when:
1) they have more EWGs
2) the carbonyl is close to the EWG (induction)
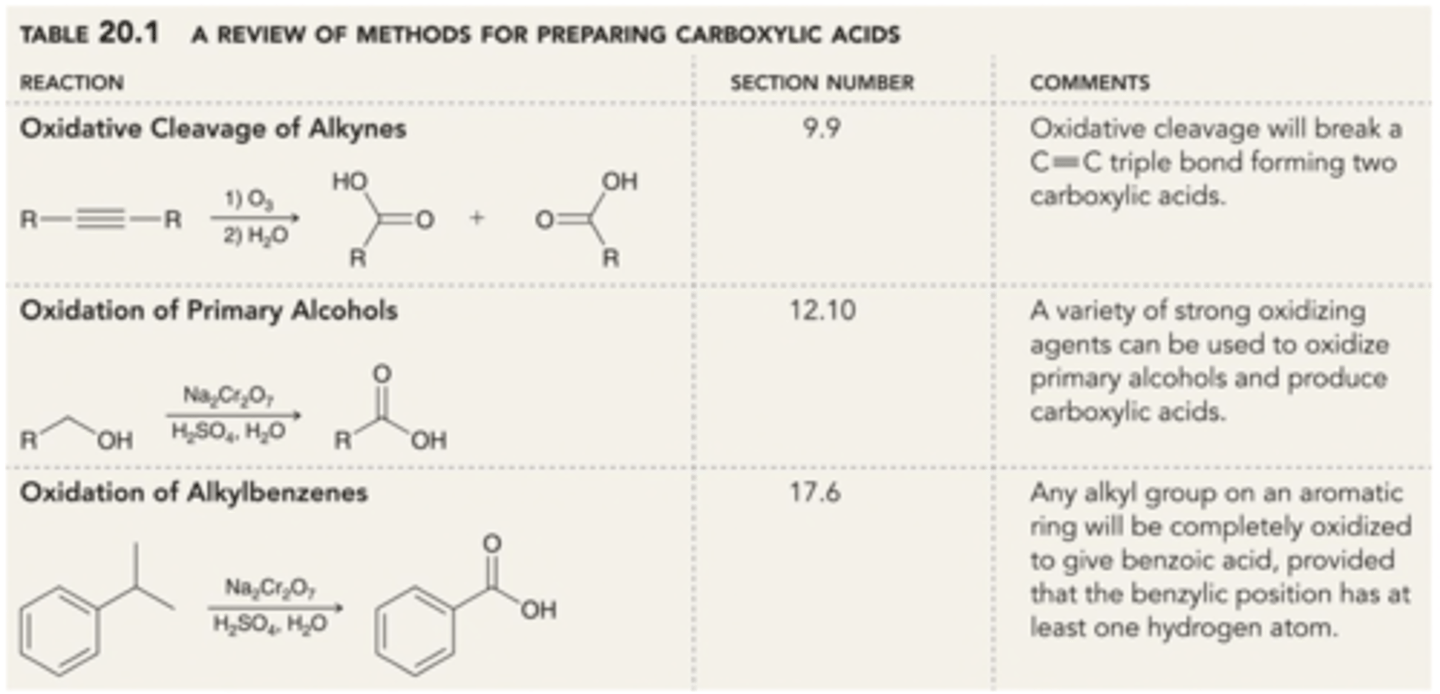
Preparation of Carboxylic Acids (Review)
1) Oxidative Cleavage of Alkynes
Alkyne → Carboxylic Acid
Reagents: 1) O3 / 2) H2O
2) Oxidation of 1° Alcohols
1° Alcohol → Carboxylic Acid
Reagents: Na2Cr2O7, H2SO4, H2O
3) Oxidation of Alkylbenzenes
Alkyl on Benzene → Carboxylic Acid
Reagents: Na2Cr2O7, H2SO4, H2O
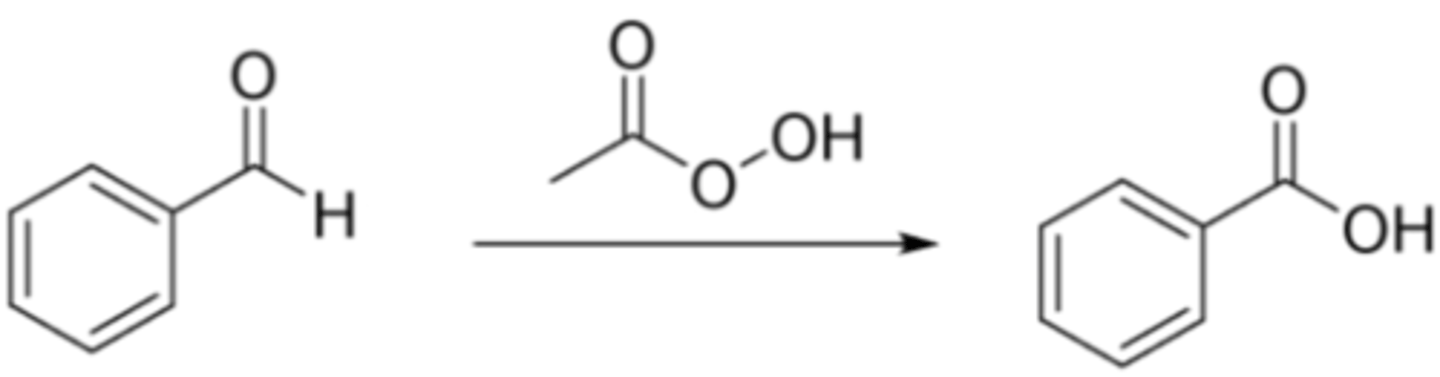
4) Preparation of Carboxylic Acids via Baeyer-Villiger Oxidation
4) Baeyer-Villiger Oxidation
Aldehyde → Carboxylic Acid
Reagents: m-CPBA (peroxy acid)
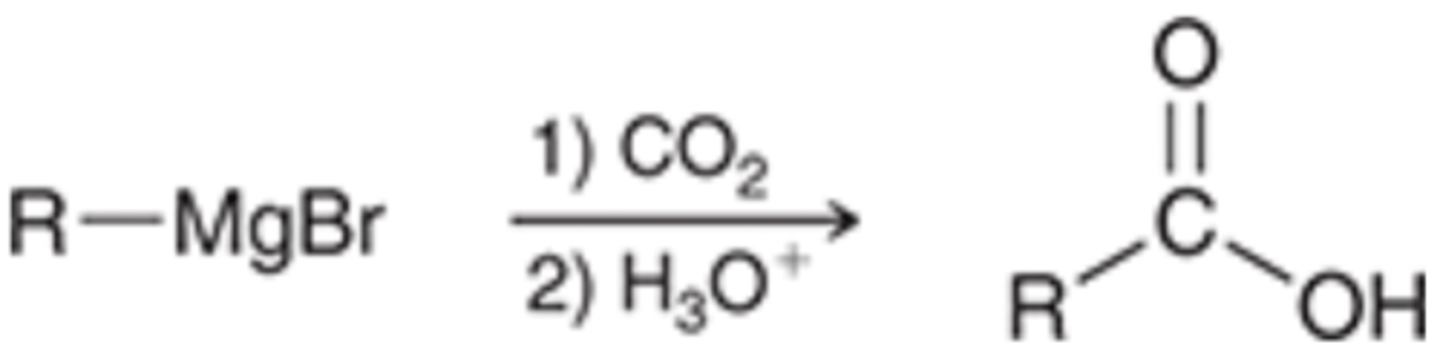
5) Preparation of Carboxylic Acids via Carboxylation of Grignard Reagents
5) Carboxylation of Grignard Reagents
Grignard Reagent (R-MgBr) → Carboxylic Acid
Reagents: 1) CO2 / 2) H30+
Reduction of Carboxylic Acids
Starting Material: Carboxylic Acid
Carboxylic Acid → Reduced to Alcohol
Reagents: 1) LAH / 2) H30+