3.3 Second Law of Thermodynamics and Entropy
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What is Entropy S ?
J/K
a measure of disorder or randomness in a system and thus the uncertainty or unpredictability pf data.
quantifies the number of ways microscopic components can be arranged.
Higher entropy means more disorder.
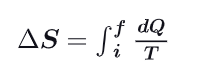
What becomes the entropy equation if the process is isothermal ?
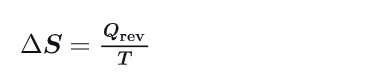
What is the second law of thermodynamics ?
if a process is applied : Consider a certain amount of gas enclosed in a cylinder–piston chamber and an external compressive force is applied on the piston to decrease the chamber’s volume
In a closed system, the entropy of the system increases for irreversible processes
It remains constant for reversible processes and never decreases

What is the cycle of carnot ?
A Carnot engine is a perfect imaginary heat engine.
It:
takes in heat from something hot,
uses that heat to move or push (it “does work”),
then throws away some leftover heat to something cold.

Describe the 4 stages of a carnot cycle (and draw the T-S diagram)
ab – Isothermal Expansion
Heat Q_H enters from the hot reservoir at constant temperature T_H
The gas expands and does work.bc – Adiabatic Expansion
No heat exchange.
The gas keeps expanding, so it cools down (from T_H to T_L).cd – Isothermal Compression
Heat Q_L is released to the cold reservoir at constant temperature T_L.
The gas is compressed.da – Adiabatic Compression
No heat exchange
The gas is compressed, so it heats up (from T_L back to T_H).
Area within loop cycle = net useful work generated by the system
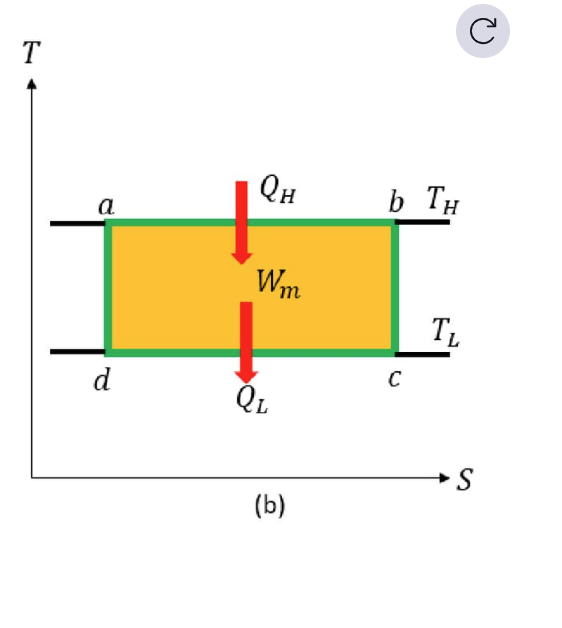
What is the relationship between heat and useful work in a carnot cycle ?
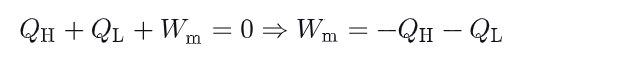
what is the relationship between Q-H, T_H & Q_L & T_L ? write the demonstration
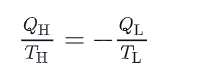
What is the fomula for the efficiency of a carnot cycle using Work and Heat exchanged ? Write the demonstration
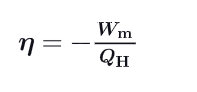
What is the formula for the efficiency of a carnot cycle using temperatures ?
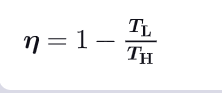
Is the efficiency formula an idealization ? What Would be missing ?
Yes, it depends of the fluid used or the internal structure and design of the engine