Unit 1.3 - Cell Membranes & Transport
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
Cell Membranes - Phospholipids
Cell membranes have two main components, phospholipids and protein.
Phospholipids are formed when glycerol is bonded to 2 fatty acids & 1 phosphate group
What is the Phospholipid/ ‘lipid’ bilayer?
Double layer of phospholipids.
What is the width of the membrane?
7/8 nm.
Why is transport across the membrane vital to the cell?
Maintain water potential
Obtain oxygen/ nutrients
Removes toxic substances/ Carbon dioxide
What is a monolayer & a bilayer?
If isolated phospholipids are placed on water, they form a monolayer with the hydrophilic heads in the water and the tails which repel water sticking out into the air.
In cells, as cytoplasm is watery & cells are bathed in watery solutions, phospholipids arrange themselves in a bilayer - hydrophilic heads next to water on both sides & hydrophobic tails in the centre.
The phospholipid head is charged.
Calculation to work out surface area of mono/bilayer
To work out the S.A of the bilayer - actual cell membrane (e.g red blood cell) when given the monolayer’s S.A you would halve it.
To work out the S.A of the monolayer from the bilayer’s S.A you would double it.
(Remember: In a monolayer, the bilayer has unfolded therefore surface area is larger)
What are Intrinsic & Extrinsic (Membrane) Proteins? + Structure
Intrinsic Proteins: span the phospholipid bilayer & some form channels through the membrane
Some are enzymes, protein channels & protein carriers
Extrinsic Proteins: found on 1 side of the phospholipid bilayer or on the surface of the bilayer
May be specific receptors, e.g. for hormones or neurotransmitters
add diagram
R Groups - Amino acids
The R groups are variable groups of amino acids.
Some R groups are polar (slight +/- charges)/ (fully) charged, so are hydrophilic.
Some R groups are non-polar/ non-charged so are hydrophobic.
Where are polar & non-polar R groups in proteins often located in the phospholipid bilayer and why?
Polar R groups - near the phosphate heads (surface of the membrane) because they are charged and interact with polar molecules.
Non- Polar R groups - near the fatty acid tails because they are hydrophobic and don’t interact with polar molecules.
Why do channel proteins often have polar R groups lining the channel?
So polar/ charged molecules can pass through.
Selectively Permeable Membranes
Cell membranes are selectively permeable. The hydrophobic layer is impermeable to polar and charged molecules & permeable to non-polar and uncharged molecules, such as oxygen, carbon dioxide & fat soluble vitamins.
Non-polar & uncharged molecules can dissolve in the fatty acids/ hydrophobic layer & cross the membrane via simple diffusion.
Channel Proteins
Channel proteins are intrinsic proteins & have specific R groups lining the pore. They can open & close to regulate movement of molecules.
Channel proteins are specific to the charged or polar molecule or ion that they transport.
These molecules cross through the membrane via facilitated diffusion if moving from an area of high to low concentration.
Carrier Proteins
Carrier proteins are intrinsic proteins with specific R groups lining them.
They can open & close or change shape to transport larger charged or polar molecules across the membrane, so can also carry out facilitated diffusion, moving molecules from high to low concentrations.
Carrier proteins can also carry out active transport, which moves charged or polar molecules from areas of low to high concentration, using energy from ATP.
What is Glycocalyx?
On the outer surface of cell membranes, some proteins & phospholipid heads have complex carbohydrate groups attached.
Glycoproteins = proteins with carbohydrates attached (glycosylated)
Glycolipids = phospholipids with carbohydrates attached (glycosylated)
Glycocalyx = all of the carbohydrates projecting outside of the cell
What are the roles of the Glycocalyx?
Cell to cell recognition
Antigens (unique to the individual) - glycoproteins
Receptors - glycolipids
What affects Cholesterol?
In the hydrophobic layer between fatty acid tails, molecules of cholesterol regulate the fluidity of the membrane.
Not enough cholesterol —> membrane too fluid
Too much cholesterol —> membrane too rigid
Fluid Mosaic Model
In 1972, Singer & Nicolson put forward the fluid mosaic model of membrane structure:
Fluid - phospholipids free to move laterally in the membrane
Mosaic - random pattern of proteins in the membrane
Mosaic evidence came from freeze fracture electron microscopy
Membrane fractured along line of least resistance (centre of the bilayer)
Images produced have speckled effect caused by exposed proteins
Fluidity evidence came from different experiments.
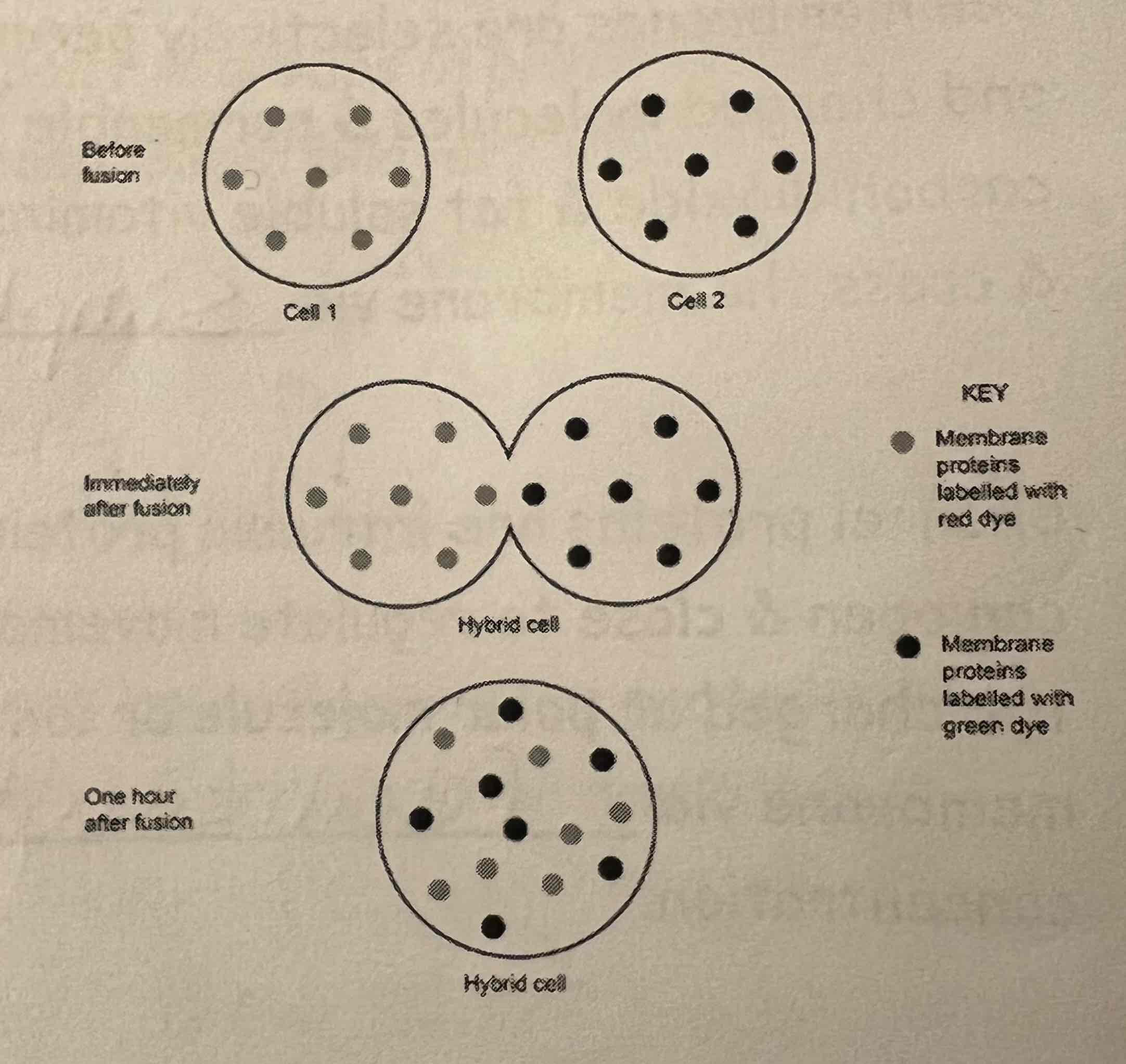
Fluidity Evidence - Examples 1 & 2
Example 1:
One cell's membrane proteins dyed one colour (e.g. red) & another cell's membrane proteins dyed a different colour (e.g. green)
Both cells fused
An hour after fusion, the differently coloured dyed proteins have dispersed evenly across the membrane
Example 2:
Attaching fluorescent markers to phospholipid or protein molecules
Bleaching an area of the membrane
Overtime the bleached area 'disappears' as the bleached & marked molecules mix
Compare Fluid Mosaic Model to Davson-Danielli’s Model of the plasma membrane
Similarities:
They both have a phospholipid bilayer with hydrophobic tails and hydrophilic heads
Hydrophobic tails are inside & hydrophilic heads are outside
They both have proteins
Differences:
No intrinsic proteins in Davson-Danielli’s model
There is an absence of glycocalyx/ glycoproteins/ glycolipids/ cholesterol in Davson-Daniellli’s model
How does fatty acid chain length affect the temperature at which the phospholipid bilayer becomes fluid?
As the number of carbon atoms in the fatty acid chain increases, the transition temperature increases.
Because the longer the chain length, the greater the intermolecular forces.
Therefore more energy is needed to break the bonds/ overcome the intermolecular forces.
Why does the presence of unsaturated fatty acids in the phospholipid increase fluidity of the membrane?
Unsaturated fatty acids contain double bonds between neighbouring carbon atoms in the hydrocarbon tails.
This produces a kink in the side chain, so there is increased distance between the chains.
This lowers the intermolecular forces, so less energy is needed to break the bonds/ overcome the intermolecular forces.
Specified Practical: An investigation into the permeability of cell membranes using beetroot - Method
Beetroot Specified Practical
Cylinders of beetroot are cut to 1cm in length - This controls the surface area of the tissue.
The cylinders are rinsed to wash away betacynine (pigment) from damaged cells.
A thermostatically controlled water bath should be used to maintain temperature of the water.
After 30 minutes, the tubes of water are agitated & the beetroot is removed.
Results of Beetroot Specified Practical
Judging colour by eye is subjective, however a trend will be seen of light to dark as temperature increases. (Semi-quantitative).
A colorimeter is more objective & gives numerical data that can be plotted on a graph.
If measuring transmission, paler colours (less pigment) gives a higher transmission (more light passes through) so the trend will be high to low as temperature increases.
If measuring absorption, the darker colour (more pigment) gives a higher absorption (more light absorbed) & the trend is low to high as temperature increases.
Ethanol & organic solvents dissolve phospholipids, so increasing concentrations increase membrane permeability.
How can a colorimeter be used to detect colour change.
Colorimetry uses a colorimeter to measure light transmission/ absorbance by a solution.
Prediction, Variables & Repeats for Experiment of Membrane Permeability
Prediction - As temperature increases, the absorption increases.
IV (change) - Temperature (oC) - 25, 35, 45, 55, 65
DV (measure) - The absorbance of external solution
CV (keep the same):
Size of beetroot cylinder - because S.A affects rate of diffusion
Amount of time beetroots are left in the water
Volume of water in the test tube - it will affect concentration of the pigment (more water = lower concentration)
Will do 3 repeats to identify anomalies, and to create a mean.
Why might a student conduct a second experiment, with a different independent variable?
To act as a control
To show that the observation/ result of the experiment was due to the independent variable, and no other extraneous variable.
Passive Transport across the cell membrane
Passive means that ATP/energy from respiration is not required - Respiratory inhibitors (e.g cyanide) that inhibit ATP production do not affect passive transport methods.
What is Diffusion & Simple Diffusion?
Diffusion: Passive, random net movement of particles from a region of high to low concentration (until equally distributed).
Simple diffusion: Passive movement of particles from a region of high to low concentration through the phospholipid bilayer.
Small, non-polar, non-charged or lipid-soluble molecules dissolve in fatty acid tails & cross the membrane by simple diffusion e.g. Oxygen, carbon dioxide & fat-soluble vitamins (A, D, E & K)
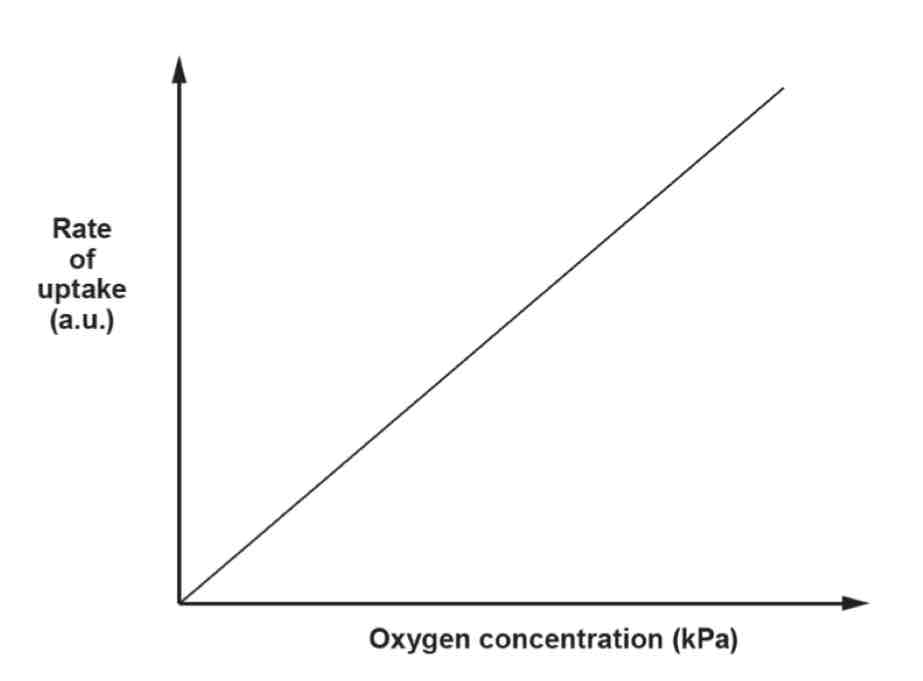
Diffusion Graph
Rate is proportional to concentration.
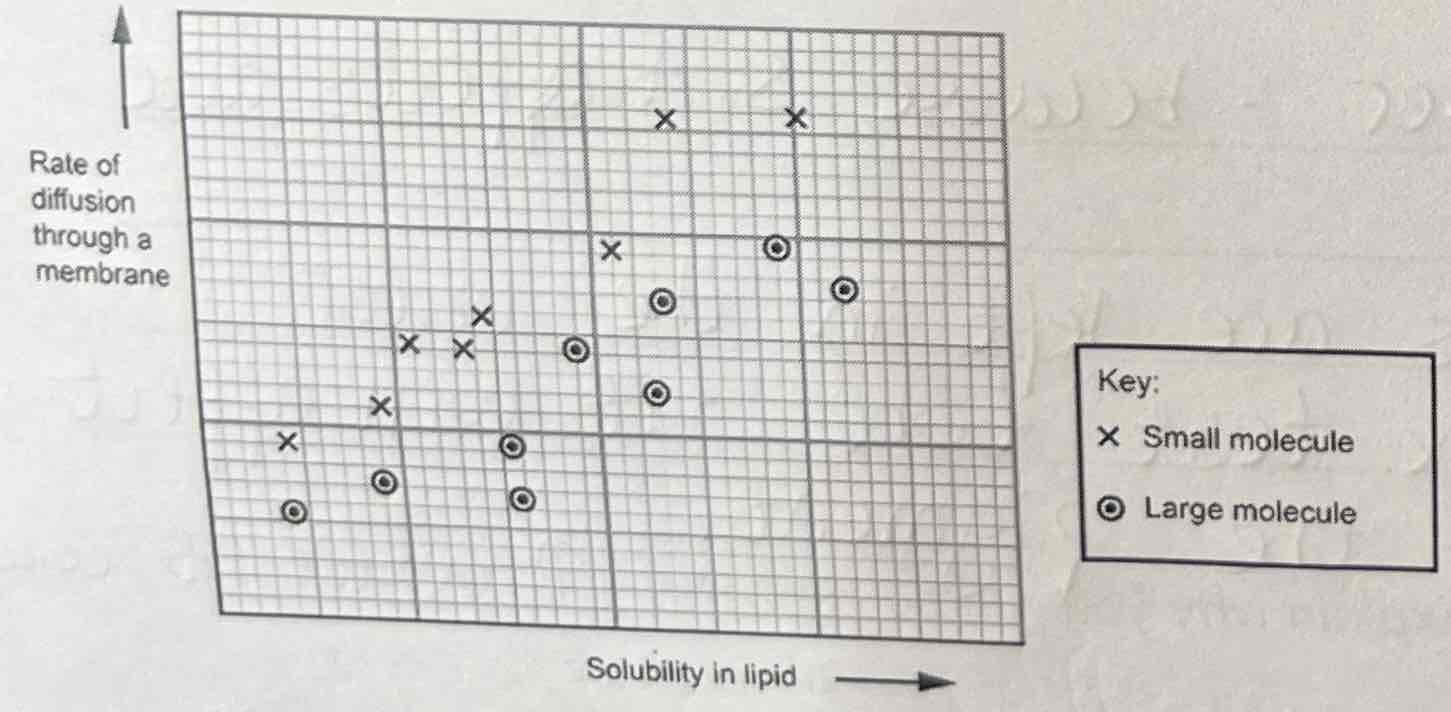
Describe & Explain the Graph
As solubility in lipid increases, the rate of diffusion through a membrane increases, because it dissolves more easily in the phospholipid bilayer.
Smaller molecules have a greater rate of diffusion because they can fit through the gaps in the phospholipid bilayer more easily.
What Factors affect the rate of diffusion?
Surface area of the membrane
Length of diffusion distance
Steepness of concentration (water potential) gradient
Temperature
Lipid soluble molecule size
Lipid Solubility
Membrane permeability can also be affected by salt concentration, presence of detergents and organic solvents (organic solvents dissolve lipids, e.g alcohol is an organic solvent)
How do acids affect membrane permeability?
Acid denatures/ changes shape of proteins (in cell membrane - which are enzymes)
Factors affecting the rate of diffusion - Surface area of the membrane
Higher surface area gives more places over which diffusion can happen & increases the rate of diffusion. Folds in the cell membrane increase the surface area.
Factors affecting the rate of diffusion - Length of diffusion distance
The shorter the diffusion distance, the faster diffusion can happen as it takes less time. Flattened cells, thinner membranes & fewer layers of cells decrease diffusion distance.
Factors affecting the rate of diffusion - Steepness of concentration gradient
The larger the difference between the high and low concentrations, the faster diffusion happens. Circulation and ventilation increase concentration gradients.
Factors affecting the rate of diffusion - Temperature
The higher the temperature, the more kinetic energy particles have & the faster they move, colliding with the membrane more frequently. This increases the rate of diffusion.
Factors affecting the rate of diffusion - Lipid soluble molecule size
The smaller a lipid-soluble molecule is, the faster it will diffuse across a membrane. (Water-soluble molecules diffuse through protein channels/ carriers so it’s size won’t affect diffusion rate).
Factors affecting the rate of diffusion - Lipid Solubility
The more lipid-soluble a molecule is, the faster it will diffuse across a membrane.
What is Facilitated Diffusion?
Facilitated diffusion: Passive movement of particles from a high to low concentration through specific protein channels or carriers.
Charged particles, polar molecules & large molecules cannot dissolve in fatty acid tails/ hydrophobic layer, so it cannot pass through phospholipid bilayer & so crosses the membrane through transport proteins by facilitated diffusion e.g. Glucose, amino acids & water
What affects the rate of facilitated diffusion?
The rate of facilitated diffusion is also dependent on the number of transport proteins.
The number of transport proteins limits the rate of facilitated diffusion, as a maximum rate of diffusion is reached when there's a high concentration of the substance that is being transported.
This means that all of the protein carriers are in use or saturated.
Comparing Channel & Carrier Proteins
Similarities between Channel & Carrier Proteins:
Hydrophilic lining
Specific
Can open & close
Differences:
Small, charged or polar molecules pass through channel proteins e.g. Na
Larger, charged or polar molecules pass through carrier proteins e.g. glucose & amino acids
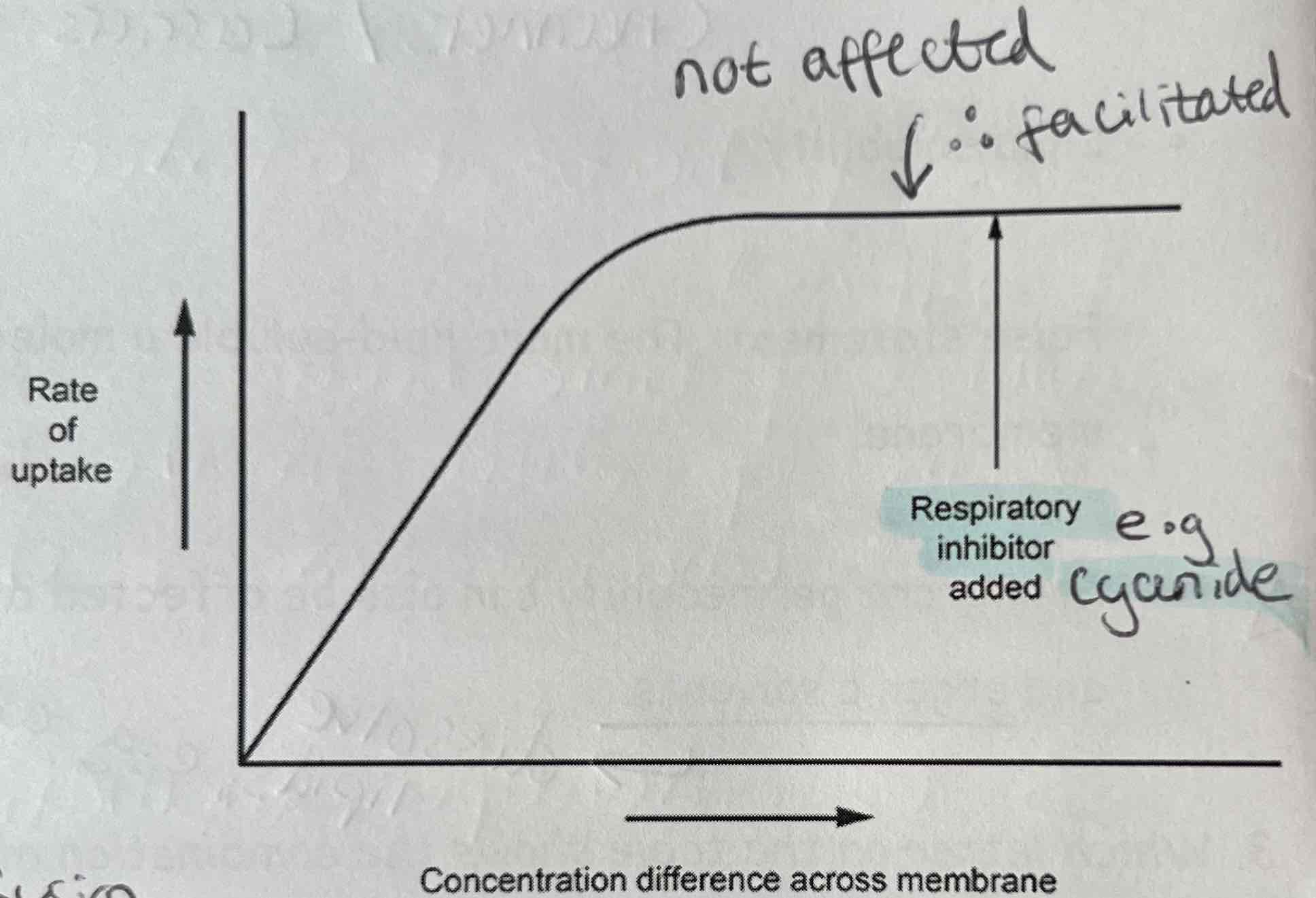
Describe & explain how concentration gradient, respiratory inhibitors & protein carriers affect facilitated diffusion rate as shown on the graph
Increasing concentration gradient increases facilitated diffusion
Respiratory Inhibitors (cyanide) don’t affect facilitated diffusion because it doesn’t need energy/ ATP
More protein carriers increase rate of facilitated diffusion (if there are not enough protein carriers, it limits the rate of facilitated diffusion)
Which factors affect facilitated diffusion?
Temperature
More specific protein carriers
Higher concentration gradient
Which factors don’t affect facilitated diffusion?
Higher respiration rate
Increased lipid-solubility
How does Temperature affect facilitated diffusion?
The higher the temperature, the more kinetic energy particles have, collisions with the protein channels and carriers happen more frequently.
Above a certain temperature, the protein channels and carriers diffuse, allowing all particles to diffuse through.
How do more specific protein carriers affect facilitated diffusion?
The more protein carriers, the greater the rate of facilitated diffusion due to more surface area (of protein channels) that the substances can diffuse through up until a point when concentration gradient is the limiting factor.
How does a higher concentration gradient affect facilitated diffusion?
The greater the concentration gradient, the greater the rate of facilitated diffusion, up until a point where the protein channels or carriers are saturated and working at maximum capacity.
Why doesn’t a higher respiration rate affect facilitated diffusion?
A greater respiration rate does not affect facilitated diffusion as it does not use ATP.
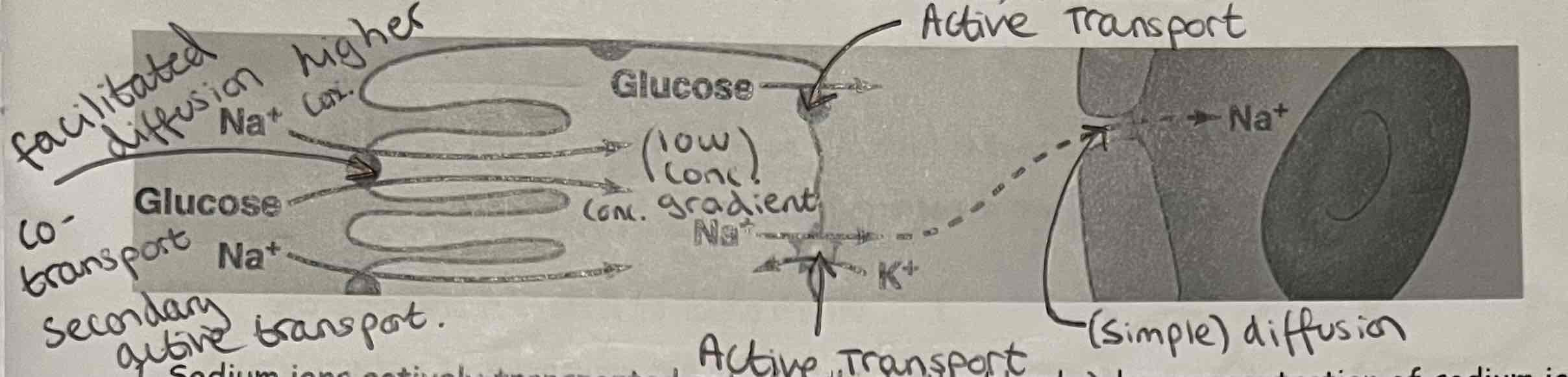
What is Co-Transport?
Co-transport: A type of facilitated diffusion where 2 different particles are transported through the same protein at the same time.
E.g. how glucose is absorbed in the ileum (small intestine) of mammals
Co-Transport + Labelled Diagrams
Sodium ions actively transported out of cell into the blood → low concentration of sodium ions inside cell
Higher concentration of sodium ions outside of the cell in the intestine lumen → sodium diffuses into the cell via a co-transport protein, with glucose
Glucose leave the other side of the cell into the blood via facilitated diffusion
(Sodium ion concentration gradient powers movement of glucose in co-transport, not ATP directly)
What is Osmosis?
Osmosis: Passive, net movement of water molecules from an area of higher water potential to an area of lower water potential across a selectively permeable membrane.
Net movement is the overall movement (particles move in both directions randomly)
What is Water Potential?
Water Potential (ψ): tendency of water to move into or out of a system by osmosis & the pressure created by the water molecules.
It depends on:
Solute concentration (solute potential)
Pressure on the solution (pressure potential)
Water Potential Examples
Water potential of pure water = 0 kPa
Water potential of typical cell = -200 kPa
Water potential of strong glucose solution = -1000 kPa
Solute potential (ψs) is the reduction in water potential due to the presence of solute molecules
All potentials have units kPa (kilopascals)
Values of Water & Solute Potentials
Water & solute potentials are negative values.
High potentials have a less negative number, e.g. -360 kPa is higher than -410kPa
Higher concentration of solute (any substance dissolved in a solvent) = lower water potential, so if glucose or ions pumped or diffuse into cell, water potential gets lower.
Water moves from higher to lower water potential.
Osmosis in animal/ plant cells
Hypertonic solution - higher concentration of solute and lower water potential than cells (low water potential —> water moves out of the cell)
Isotonic solution - same concentration of solute & same water potential as cells (no net movement —> water moves in and out of the cell at the same rate)
Hypotonic solutions - lower solute concentration & higher water potential then cells (high water potential —> water moves into the cell)
What is Haemolysis & Crenation?
Haemolysis (red blood cell bursting as no cell wall) - bursts in hypotonic solutions as water moves into the cell.
Crenation (wrinkled appearance of animal cells) - happens in hypertonic solutions as water moves out of the cell.
What are Red Blood Cells used to demonstrate? + Issues
Red blood cells are often used to demonstrate osmosis in animals because they are suspended in plasma, not connected.
Following haemolysis, red pigment (haemoglobin) is released into surrounding solution - if removed by centrifugation, depth of colour is measured with colorimeter.
Issues:
Blood could be infected
Platelets cause blood clots when removed from animals
Each blood cell in a sample has a different water potential, so the cells burst over a range of solute concentrations
Graph - How to answer a Describe & Explain Question
Describe:
Use labels of each axis and state the trend (i.e as X increases, Y decreases)
If more than one mark, give values from graph (i.e significant info.)
Explain:
Use own biological knowledge to explain results (i.e why does this happen?)
How do you work out the overall salt concentration of red blood cells from a graph?
Draw a line at 50% (average), up to the line/ curve, and draw a line down to the x axis (read value)
What is Pressure Potential?
Pressure Potential: resistance of the cell wall to water entering via osmosis
Pressure potential is always a positive value.
Osmosis in Plant Cells
In plant cells, the water potential depends on the solute concentration in the cytoplasm & vacuole and the pressure potential generated by the cell wall.
As cells become turgid, the cytoplasm pushes against the cell wall, which resists further expansion, exerting a pressure on the cytoplasm & preventing further entry of water.
What equation expresses the relationship between the solute, water and pressure potentials?
Remember: Pressure potential is a positive number, solute and water potentials are negative

Plant Cells Osmosis - Solution with Higher Water Potential (Hypotonic)
When plant cells are immersed in solutions with a higher water potential than the cell contents; water enters by osmosis & the cytoplasm and vacuole expand. The plant cell becomes turgid.
When it is not physically possible for any more water to enter the cell, the water potential of the cell is 0 kPa.
This means the solute potential & pressure potential cancel each other out.
Plant Cells Osmosis - Solution with Lower Water Potential (Hypertonic)
When plant cells are immersed in solutions that have a lower water potential than the cell contents, water leaves the cells by osmosis.
The vacuole and cytoplasm shrink because of the loss of water & the cytoplasm pulls away from the cell wall. Down a microscope there is a visible gap between the cell membrane & cell wall.
The cell membrane pulled away from the cell wall is known as plasmolysis & the cells are termed plasmolysed & the cells are said to be flaccid.
As the cell wall is fully permeable, the solution passes through it & therefore the water potential on both sides of the cell wall are the same.
Water Potentials of Cells
In a tissue, each cell has its own water potential:
Cells plasmolyse at different solute concentrations
Incipient plasmolysis: where 50% of the cells are plasmolysed is the point when the water potentials of solution & tissue are equal (equilibrium is reached)
When cells are plasmolysed the pressure potential is 0 kPa; the water potential & solute potential of the cells are equal.
Features of Turgid Cells
Turgid Cells: (Cell membrane/ cytoplasm pushed against cell wall)
Water Potential = 0 kPa
Therefore, 0 kPa = pressure potential + solute potential
Therefore, - (negative) solute potential = pressure potential (equal and opposite)
What is Incipient Plasmolysis?
Incipient Plasmolysis: (Cell membrane/ cytoplasm beginning to pull away from cell wall)
Pressure potential = 0 kPa
Therefore, water potential = 0 kPa + solute potential
Therefore, water potential = solute potential
Features of Plasmolysed Cells
Plasmolysed Cells: (Cell membrane/ cytoplasm completely pulled away from cell wall)
Pressure potential = 0 kPa
Water can move via osmosis through the cell wall, so, water potential becomes bigger than the solute potential
Measuring Incipient Plasmolysis
Measuring incipient plasmolysis:
Plant cells placed into solutions with varying solute potentials
Cell observed under microscope & number of plasmolysed cells & number of total cells counted
When percentage of plasmolysed cells is 50% incipient plasmolysis is reached
Measure value at 50%, use conversion table and give answer in range e.g 0.36moldm-3 is between 0.35 and 0.40, so solute potential is between those kPa values.
What is Active Transport?
Active transport: moves substances from low to high concentrations (against a concentration gradient) using specific protein carriers & energy from A TP.
What affects the rate of respiration?
The rate of respiration is affected by respiration rate:
Aerobic respiration uses oxygen - more oxygen available → faster respiration rate & active transport
Increased temperatures → faster respiration rate & active transport
Why does Cyanide prevent active transport?
Cyanide is a respiratory inhibitor that binds cytochrome in the electron transport chain (part of respiration), preventing ATP production, therefore it stops active transport as it requires ATP.