BME 312 Study Set: Natural Polymers Terms & Definitions
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
polysaccharides
- mostly found in plants
- long polymer chains composed of monosaccharide units bound tg by glycosidic linkages
- range in structure from linear to highly branched
glucose
- simple sugar, most abundant monosaccharide, starts from starch
- found in blood as a sugar
- mainly made by plants and algae
- starch: 70% branched, insoluble in cold water, hygroscopic (absorbs moisture from air), used in bone replacement implants, bone cements, drug delivery, tissue scaffolds
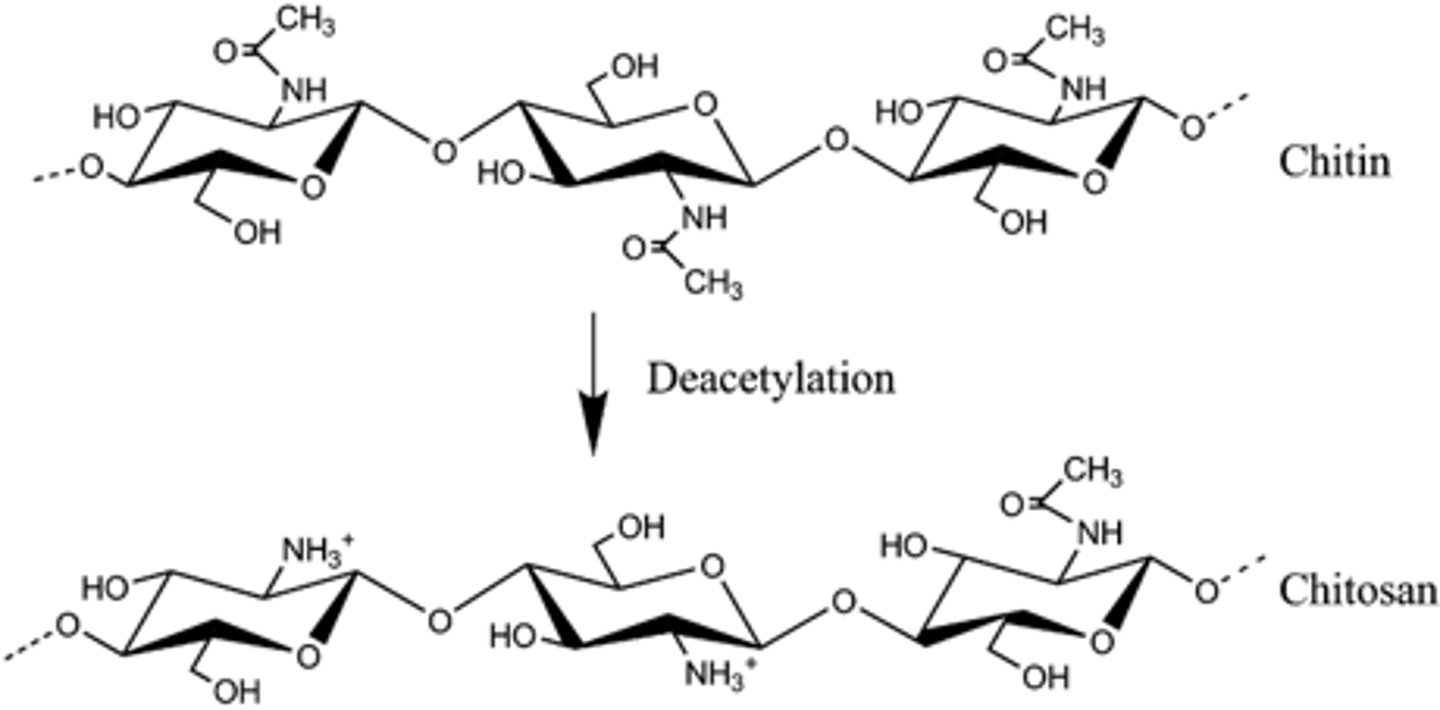
chitin
- natural polysaccharide from exoskeletons of crustaceans and insects, cell walls of some bacteria, and fungi such as mushrooms
- derivative of cellulose, insoluble in common solvents which causes difficulty in processing, not used much in applications
- highly crystalline, strong, rigid, linear polymer
- shows solubility in some concentrated acidic solvents
chitosan
- derived from chitin, much easier to dissolve in natural solvents
- linear polymer that consists of repeating units of N-acetylglucosamine and glucosamine monomers linked by glycosidic bonds
- degree of deacetylation and MW are both known to affect the properties of derived materials
- the amino and hydroxyl groups present in the chitosan chains facilitate the chemical modification and so the tunability of its structure and functional properties
- controlled deacetylation is used to produce chitosan
steps to create chitosan
1. deproteination
2. demineralization
3. discoloration
4. deacetylation
chitosan properties
- biocompatible and biodegradable
- shorten the time of wound healing and the rebuilding of connective tissue
- antioxidant, immunity enhancing, antimicrobial, analgesic, hemostatic
- minimal rxns occur
- almost soluble in water (much more than chitin)
- exhibits low toxicity and biodegradation
- used as intraocular lens material bc of its oxygen permeability and has been found to expedite blood clotting
wound healing and chitosan
1. hemostasis: coagulation (blood clotting), promotes platelet aggregation, fibrinogen is transformed into insoluble fibrin that forms clots, blood vessels constrict
2. inflammation: bacteria and necrotic tissue are cleared by inflammatory cells (rids site of bacteria - antimicrobial)
3. proliferation: epithelial cells proliferate and migrate to form epithelial tissue to cover the wound in this stage (recovery)
4. skin remodeling: fresh epidermis and dermis will generate to finish skin repair procedure
antimicrobial activity of chitosan
- inhibition of the mRNA and protein synthesis via penetration of chitosan into the nuclei of the microorganism
- formation of an external barrier which deprives bacteria from nutrients and growth
- antibiotics prevent bacteria: chitosan enters nucleus which deprives bacteria from nutrients hindering its growth
anti-tumor activity of chitosan
- low MW water-soluble chitosans and oligochitosans might be useful in preventing tumor growth, partly through enhancing cytotoxic activity against tumors as an immunomodulator
- degradation products are nontoxic: non-immunogenic and non-cancerogenic
chelation
- the ability to absorb metal ions
- chitosan has the highest chelating ability compared to other natural polymers
- this phenomena has been used for water filtration and other environmental applications (removal of dyes, etc)
- metals in body can cause toxicity so chitosan can absorb metals in body due to high affinity of metals
- chelation therapy (GSW victims)
- chitosan and metal ions (Zn, Zr, Ag) have the properties of disinfection and bactericide
pH and chitosan
- pH can be altered to effect metal absorption
- acidity can alter chelation
- solubility of chitosan at physiological pH tends to be low
- use of magnetic resins helps with removal of some metals from aqueous solutions
pullulan
- linear homopolysaccharide of glucose
- secreted primarily by strains of the fungus aureobasidium pullulans
- used in food industry, pill coating, listerine breath strip (hydrophilic)
- formed by polymerization of either panosyl or isopanosyl
- production began in Japan, process is cumbersome bc of removal of melanin from aureobasidium so production cost is high
- very high HW so it needs to be processed, not good for applications (issue)
pullalan properties
- very flexible
- allows fiber forming and distinct films
- their films and fibers resemble synthetic polymers
- hydrophilic: dissolves in water (water can be used as solvent)
- non-hydroscopic: doesnt absorb moisture
- adhesive properties (depending if placed with cells)
- oxygen impermeable properties
- odorless, tasteless, and edible: used as filler in drinks and sauces
- low viscosity: stable to heating (easy to work with), high temp, changes in pH
- when dissolved in water, it is not porous so water is not let into the structure
methods to alter properties of pullulan
- reduce its water solubility by esterification or etherification
- hydrogenation increases the stability of pullulan
- carboxylation enhances solubility in cold water
- copolymerization can change its function and ability
clinical applications of pullulan
- pharmaceutical coatings: sustained release, drug delivery
- wound healing
- vaccines
- tissue engineering
limitation of pullulan
- price $ of production has limited its demand for usage
- due to high MW and need for further processing
alginate
- naturally occurring brown seaweed extracted from brown algae
- extract is filtered and either NaCl or CaCl2 is added to the filtrate to precipitate alginate
- after further purification, water-soluble sodium alginate powder is produced
- relatively low cost to produce
- whole family of linear copolymers containing blocks of G and M residues
- G block alters properties of material
- (GGGGG) (MMMM) and (GMGMGM)
alginates (G block)
- site of cross-linking with divalent cations (Ca+2) to form hydrogels
- composition sequence, G-block length, and MW are critical factors of physical properties of alginate and its resulting hydrogels
- M block has been seen to cause toxicity
alginate properties
- mech properties are enhanced by increasing length of G-block and MW
- alginate sources provide polymers w a range of chemical structures
- high [ ] of G-blocks yields gel to have high stiffness
- biocompatibility
- low toxicity
alginate applications
- wound healing: alginate based bandages provide a moist environment (produced by ionic cross-linking with Ca+2 to form a gel, followed by processing to form freeze-dried porous sheets
- delivery of bioactive agents
- pharmaceutical: thickening, gel forming, and stabilizing agents
- drug delivery in low MW drugs
- sustained release of drugs if loaded into the alginates
alginate hydrogel
- physiochemical properties are dependent on the cross-linking type and cross-linking density, MW and chemical composition of the polymer
alginate biocompatibility
- high M content alginates were found to be immunogenic and more potent than G alginates
- disparity is due to impurities in the alginates (heavy metals, endotoxins, and proteins)
study with pullulan and ciprofloxacin (antibiotic)
- goal is to develop nanospheres encapsulated w antibiotics to use in contact lenses
- nanoparticle has a high SA to volume ratio so good for drug delivery (sustained release)
- these nanoparticles can be placed at the site of infection to limit drug side effects
- synthesis of copolymer: hydrolysis of pullulan, PCL nitrophenyl carbonate (PCL is attached to intermediate product), combined pullulan and PCL
- prep of nanoparticles: copolymer + DMSO (nontoxic solvent) + ciprofloxacin and water, use dialysis to remove DMSO and impurities, filter then freeze dry