Cell Metabolism (theory)
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Metabolism (definition)
The sum of all the chemical reactions that occur in an organism
Anabolic reaction (definition)
chemical reactions that involve the building up of molecules to form more complex molecules e.g. photosynthesis, DNA polymerase
Catabolic reactions
chemical reactions in which complex molecules are broken down into simpler molecules e.g. respiration, amylase
Catalyst
speeds up a reaction without being used up in the reaction
Enzyme (definition)
protein catalyst, which speeds up chemical reactions without being changed permanently by them
Name two enzymes and their functions
Amylase (catabolic), breaks starch down into maltose. DNA polymerase (anabolic) repairs damaged DNA
Substrate
The substance that the enzyme acts on
product
The substance formed by the reaction of the enzyme and the substrate
Enzyme shapes
Enzymes have specific shapes to fit the substrate, enzymes have complex, 3D shape in order to fit
Enzyme Reactions (Reversible? Effect of Activation Energy?)
Enzyme controlled reactions are reversible. Enzymes lower the activation energy needed to start the reaction
Inhibitors
chemicals that attach to enzymes and destroy its shape (denatured)
enzyme specificity
Most enzymes react with only one substrate. As the shape of the active site of the enzyme only fits with a certain substrate. If the shape of the active site is altered it will affect the enzyme's efficiency.
What affects an enzyme's activity/shape?
pH, temperature
The effect of pH on enzyme activity
Enzymes work within a certain pH range. The optimum (ideal) pH tends to be 7. Outside the optimum range the enzyme's activity falls (due to the enzyme's shape deteriorating and becomes denatured)
Denatured (definition)
When the active site of the enzyme loses its shape and cannot work anymore, this is often permanent
The effect of temperature on enzyme activity
At 0 degrees the cell contents becomes solid so enzyme cannot work. As the temp. increases, so does the rate of reaction. When the shape of the enzyme is lost (usually 50 degrees+) the enzyme is denatured
immobilised enzymes (definition)
enzymes that are attached/fixed to each other or to an inert material
Benefits of immobilised enzymes
reusable, more efficient, produces a clean product (essentially the product won't have the enzyme in it so it doesn't need to be separated manually)
Methods of immobilising an enzyme
Adsorption (enzymes are physically attached to inactive supports, e.g. glass beads), enzymes can be trapped in gel, e.g. Sodium Alginate. It lets the substrate enter and the product exit while the enzyme can't leave
Uses of immobilised enzymes
Food industry (glucose isomerase used to convert glucose into fructose in fizzy drinks, as fructose is sweeter but more expensive), Medicine (convert penicillin to different forms which allows the making of new antibiotics that may kill a wider range of bacteria
Bioprocessing (definition)
Use of enzyme-controlled reactions to produce a product. Immobilised enzymes are used in bioprocessing
Bioreactor
A vessel in which cells/organisms of enzymes are placed to manufacture specific products
Enzyme Active Site
the part of the enzyme that combines with the substrate. Active site will change its shape slightly when it joins the substrate. Known as the Induced Fit Theory. Previously thought that the active site was a fixed shape (lock and key theory)
Induced Fit Theory
Substrate joins the active site of the enzyme. The active site changes shape slightly. The substrate and enzyme join to make the enzyme-substrate complex. The products detach from the active site. Active site returns to original shape and can now work again
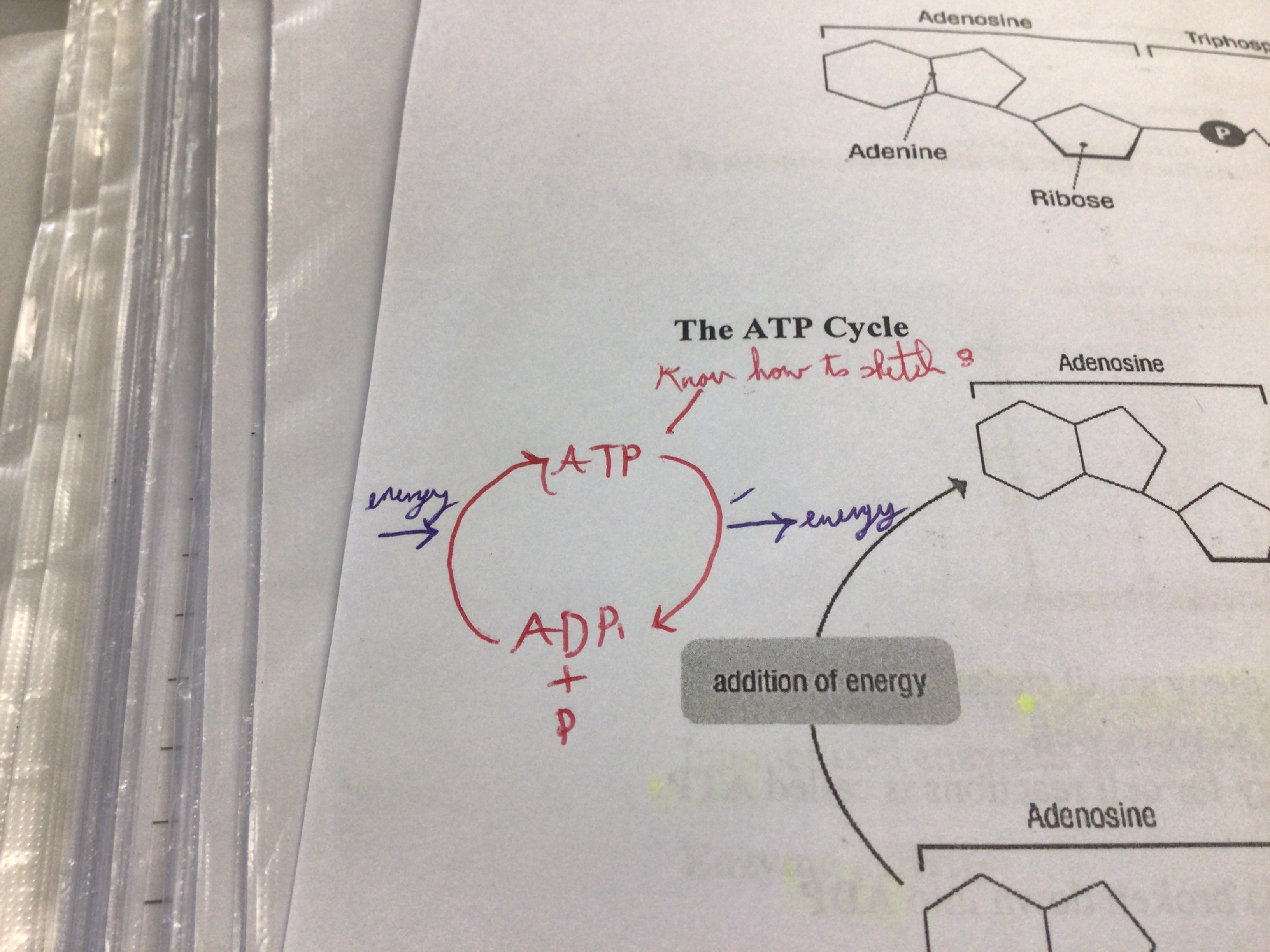
Energy Background (explanation)
Metabolic reactions take place in many small steps. Steps need small amounts of energy to work. Molecule that transfers energy, ATP. When energy is needed ATP is broken down to ADP and phosphate. This releases energy. ADP and phosphate can be re-formed back to ATP but needs energy.
When is ATP produced?
respiration and photosynthesis
What does ATP stand for?
adenosine triphosphate
What does ADP stand for?
adenosine Diphosphate
Chemical reactions for energy carriers
ATP + water--->ADP + P + energy, ADP + Energy + P--->ATP + water
Phosphorylation (definition)
process of adding a phosphate group
Draw the ATP cycle in Sketchbook
...
NAD+ and NADH
Catabolic reactions, energy and hydrogen ions are released., when NAD+ accepts these ions it becomes NADH
NADP+ and NADPH
anabolic reactions, hydrogen ions needed to make more complicated chemicals. NADPH is used to add hydrogen to the substrate. In photosynthesis NADP+ is found in chloroplasts. Light energy used to make NADPH.
Advantages of ATP over glucose
Releases energy in suitable quantities. Energy is available for immediate use. Easily recycled (ATP cycle)
Low energy chemicals (photosynthesis)
ADP, NADP+
Low energy chemicals (respiration)
ADP, NAD+
High energy chemicals (photosynthesis)
ATP, NADPH
High energy chemicals (respiration)
ATP, NADH