Mass Spectometry
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Diagram of mass spectrometer
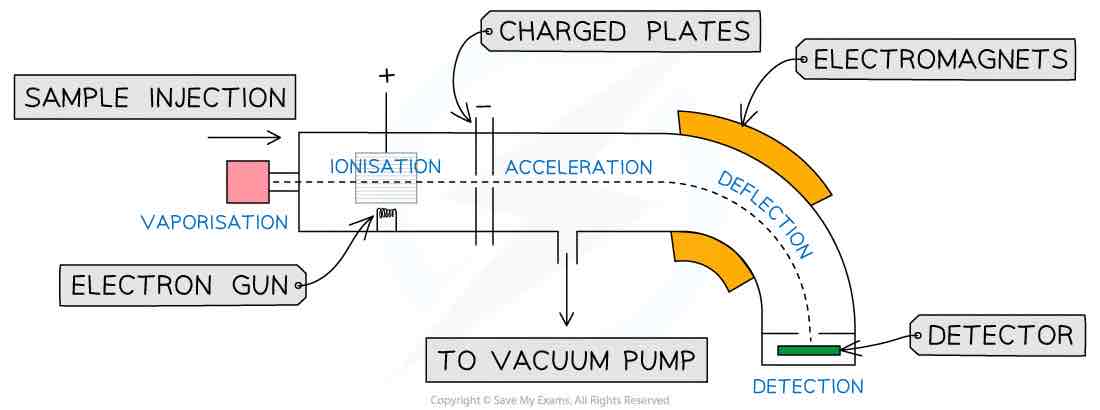
Parts of mass spectrometer (4)
High voltage/electron gun
Magnetic field
Vacuum pump
Detector
Stages of mass spectrometry (4)
Ionisation
Acceleration
Deflection
Detection
What happens in ionisation
Element is vapourised
High energy electrons fired at sample to knock electrons from shell to ionise it
What happens in acceleration
Negatively charged electromagnetic field
Accelerates positively charged ions
What happens in deflection
Electromagnetic field
Particles with higher m/z ratio deflect the most
Heavier particles are deflected less
What happens in detection
Only particles deflected a certain amount reach the detector
Causes a small current - electrons transfer from detector to positive ions to make them atoms again
Size of current is proportional to abundance of species
Graph for mass spectrum for chlorine
Cl-35 (75%)
Cl-37 (25%)
Cl35-Cl35 = 70
Cl35-Cl37 = 72 (x2)
Cl37-Cl37= 74
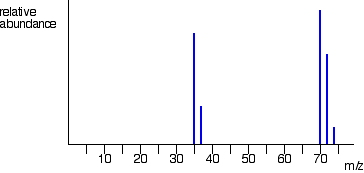
Graph for mass spectrum of bromine
Br79 = 50%
Br81 = 50%
Br79-Br79 = 158
Br79-Br81 = 160 (x2)
Br81-Br81 = 162
