Orgo 1: Functional Groups, IR Spectroscopy, Bond Length, Degrees
0.0(0)
0.0(0)
Card Sorting
1/24
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
1
New cards
Single bond length
is typically about 1.54 Å for carbon-carbon bonds, representing a sigma bond formed by the overlap of orbitals.
2
New cards
Double bond length
is usually around 1.34 Å for carbon-carbon bonds, indicating the presence of one sigma and one pi bond.
3
New cards
Triple bond length
is approximately 1.20 Å for carbon-carbon bonds, consisting of one sigma bond and two pi bonds.
4
New cards
Tetrahedral bond
109.5 degrees
5
New cards
Trigonal Planar
120 degrees
6
New cards
Linear
180 degrees
7
New cards
Alkane
Not functional group (C—H), (C—C)
8
New cards
Alkene
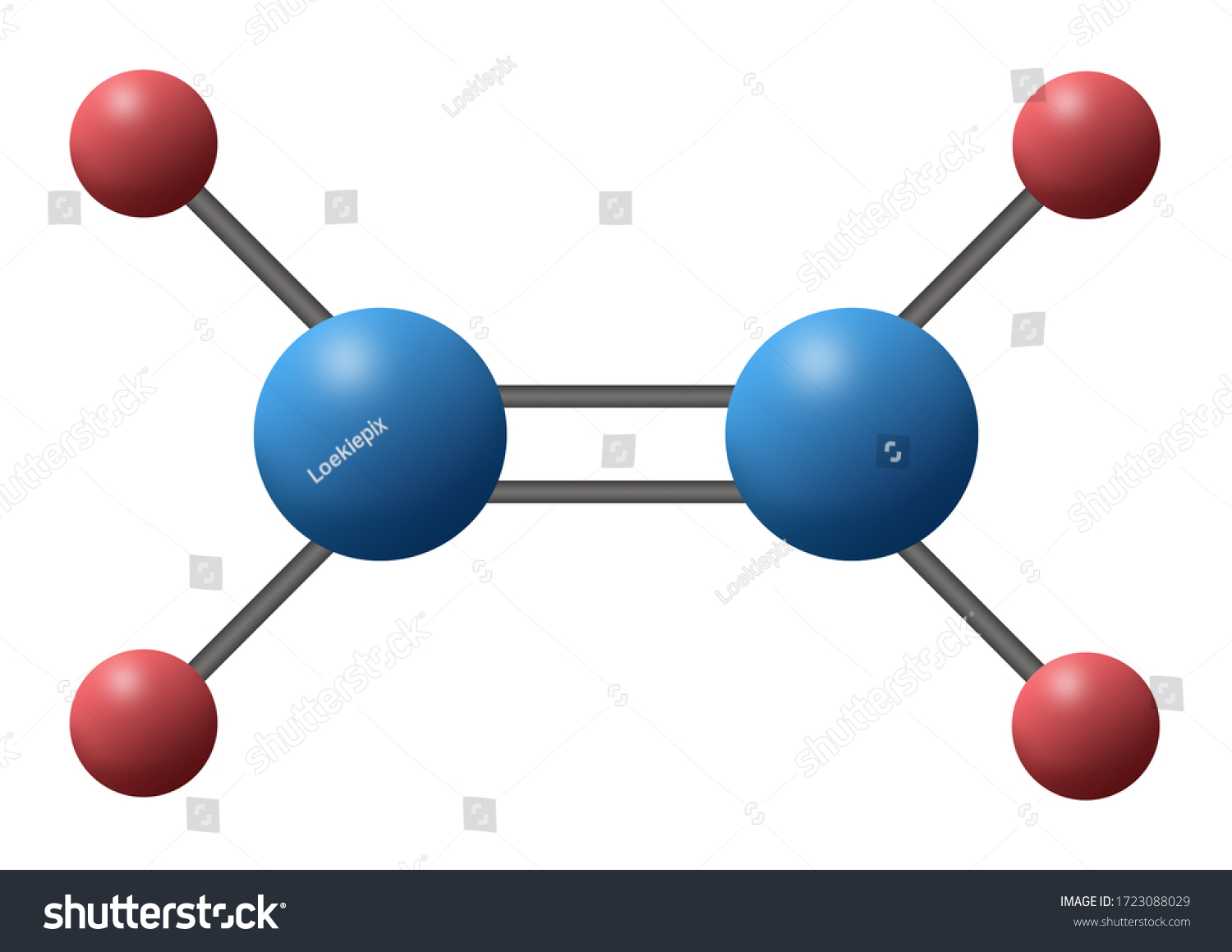
9
New cards
Alkyne
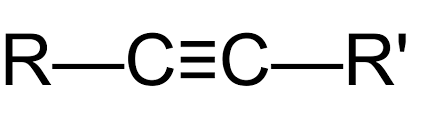
10
New cards
Aromatic
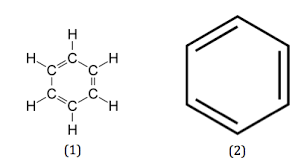
11
New cards
Haloalkane
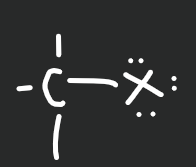
12
New cards
Alcohol
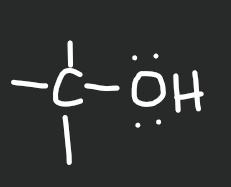
13
New cards
Ether
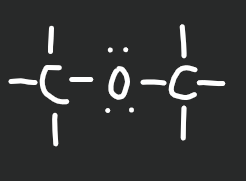
14
New cards
Amine
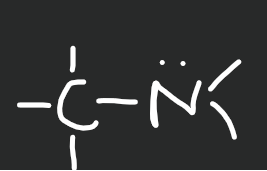
15
New cards
Aldehyde
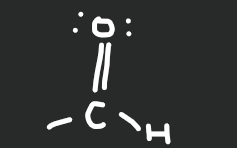
16
New cards
ketone
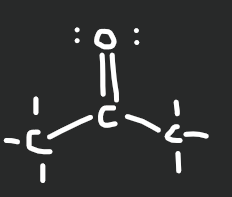
17
New cards
carboxylic acid
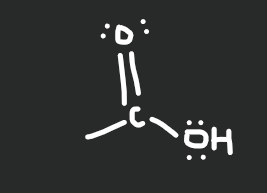
18
New cards
ester
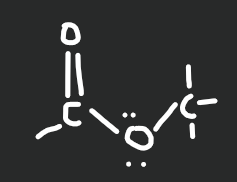
19
New cards
amide
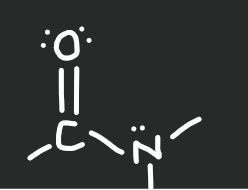
20
New cards
nitrile
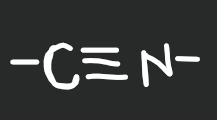
21
New cards
O-H and N-H range
around 3500
22
New cards
C-H range
around 3000
23
New cards
Triple bond range
around 2100-2260
24
New cards
C == O
around 1800
25
New cards
C==C
around 1600