EXP 4: ASSAY OF ACETAMINOPHEN TABLETS USING HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
CHROMATOGRAPHY
Separation technique in which the components of a sample are distributed between the two phases.
The separation occurs due to the
components that would react differently on distinct phases.
STATIONARY PHASE
- layer or coating on the supporting medium which interacts with the analytes.
MOBILE PHASE
- carries the solute across the stationary phase
Normal
Polar compounds are highly ADSORBED
Normal
stationary
polar
Normal
mobile
non-polar
Reverse
Non-polar compounds are highly ADSORBED
Reverse
stationary
non polar
Reverse
mobile
polar
ADSORPTION
Separation involves competing interaction between adsorption at the stationary phase and dissolution in the mobile phase
Surface binding
Compounds are separated based on their ability to be adsorbed or to stick to your stationary phase vs. how well it would dissolve in your mobile phase
ADSORPTION
USUALLY FOR
TLC and Normal phase HPLC
PARTITION
Particles are separated on the components of the solvent system
Based on the differences of solubility/partitioning between two immiscible phases (the liquid stationary phase coated with on a solid support and the mobile phase that would or is intended to contain the liquid or the gas)
PARTITION
usually for
reverse phase HPLC and Gas Liquid Chromatography
ION-EXCHANGE
Separation is based mainly on differences in the ion exchange affinities of the sample components
Differences in charge interaction between the analyte and the charge group on the stationary phase
ION-EXCHANGE
usually for
separation of amino acids, proteins, and nucleotides
Paper Chromatography
Simplest and Cheapest
More for identification = qualitative
Thin-Layer Chromatography (TLC)
Identification and Purity testing = qualitative but sometimes, quantitative (by calculating the rf value)
Identification - once the mobile phase travels, we could spray some reagents. If the reagent reacts, we could identify based on the classification.
Column Chromatography
More on separation/purification = qualitative
Gas Chromatography (GC)
Intended for analysis of residual solvent
Sample must be volatile
Qualitative and Quantitative
High-performance Liquid Chromatography (HPLC)
We try to assay drug impurities and dissolutions.
Qualitative and Quantitative
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
determine whether the data would be of high resolution, accuracy, and reproducibility.
widely applied to pharmaceutical, biological, & food analysis.
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
stationary phase
Solid (usually silica)
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
mobile phase
Liquid Solvent system
Would flow under high pressure
Isocratic
Same type of solvent
Gradient Elution
The technique of continuously changing the solvent composition during the chromatographic run
speeds up analysis
improves the resolution for rediluting compounds
Chromatographic Column
“column”: includes
stainless steel,
lined stainless steel
Polymeric columns,
packed with a stationary phase.
lined with stainless steel:
“If you want unwanted interactions”
polymeric columns:
Special column for ion-exchange, gel permeation, or metal-free environment
LENGTH & INNER DIAMETER OF THE COLUMN
affects the separation, and therefore, typical dimensions are included in the individual monograph.
LENGTH & INNER DIAMETER OF THE COLUMN
↑ length
= better resolution but longer analysis time = ↑ back pressure (resistance)
LENGTH & INNER DIAMETER OF THE COLUMN
↓ length =
faster analysis time but poor resolution
LENGTH & INNER DIAMETER OF THE COLUMN
narrow inner diameter
= ↑ efficacy & ↑ sensitivity
LENGTH & INNER DIAMETER OF THE COLUMN
wide inner diameter =
↑ capacity for the sample; too many separations would take a long time
PARTICLE SIZE OF STATIONARY PHASE
Larger particle size:
lower resolution & pressure
PARTICLE SIZE OF STATIONARY PHASE
Smaller particle size:
higher efficacy & resolution
Apparatus: Liquid Chromatogram
Reservoir containing the mobile phase
Pump to force the mobile phase through the system at high pressure
Injector to introduce the sample into the mobile phase
Chromatographic column (contains stationary phase)
Detector
Data collection device
Degassing Unit
To remove dissolved air (gases such as oxygen) from mobile phase (b)
Solvent Delivery Pump
to deliver the mobile phase at a constant flow rate and pressure
Sample Vial
To store standard solution or sample solution
Column
To separate each compound contained in the sample
Column Oven
To keep the temperature constant (d)
Detector
To detect the eluted compound(s) from the column (a)
Workstation
The signal from the detector is processed and the chromatogram is displayed (f)
does the data processing, which generates the chromatogram showing the peaks and corresponding compounds
Drain
Waste compounds exit the detection
Procedure
Equilibrate the column and detector with mobile phase at the specified flow rate until a constant signal is received.
Inject a sample through the injector or use an autosampler.
Begin the gradient program.
Record the chromatogram.
Analyze as directed in the monograph.
Retention Time
for identification. More on Qualitative
Peak height
- account for the number of compounds in the sample. More of Quantification
CHROMATOGRAM
A graphical representation of the detector response, concentration of analyte in the effluent, or other quantity used as a measure of effluent concentration versus effluent volume or time.
RETENTION TIME (tR )
is the time elapsed between the injection of the sample and the appearance of the maximum peak response of the eluted sample
AREA UNDER A PEAK
It is a measure of the concentration of the compound it represents.
analysis formula
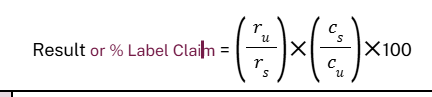
ACCEPTANCE CRITERIA
90.0% - 110.0% on the dried basis
Solution A:
1% (v/v) glacial acetic acid in water
Solution B:
Methanol