Chemistry Homologous Series
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
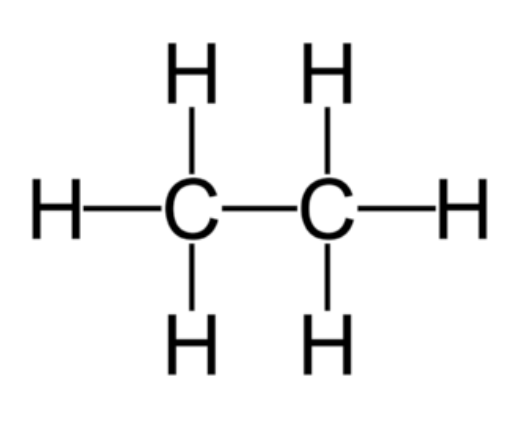
Alkanes
Saturated hydrocarbons. All single bonds.
General formula: CnH2n+2
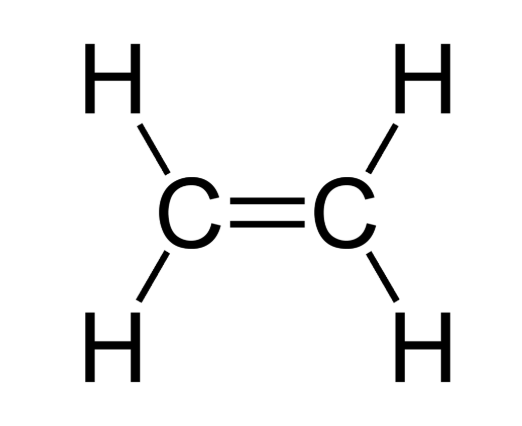
Alkenes
Unsaturated hydrocarbons. Contain at least one double bond.
General formula: CnH2n
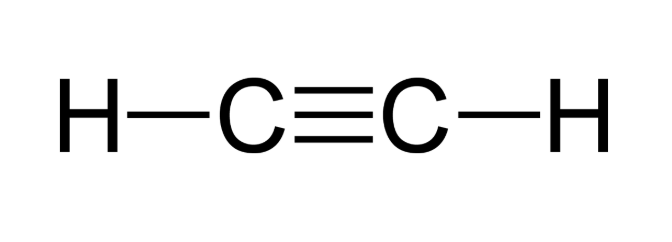
Alkynes
Unsaturated hydrocarbons. Contain at least one triple bond.
General formula: CnH2n - 2
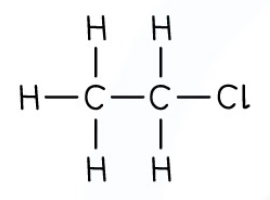
Halogenoalkanes
General formula: CnH2n + 1X
X represents a halogen.
Functional group: -X
The halogeno group.
Nomenclature: The prefix of the molecular name is the halogen i.e. chloro-, bromo- etc.
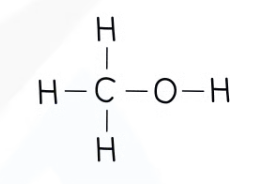
Alcohols
General formula: CnH2n + 1OH
Functional group: -OH
The hydroxyl group.
Nomenclature: alkan + ol

Primary alcohol
One carbon bonded to the functional group carbon.
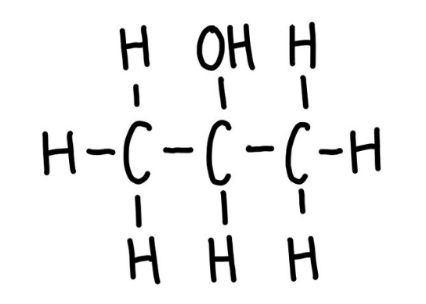
Secondary alcohol
Two carbons bonded to the functional group carbon.
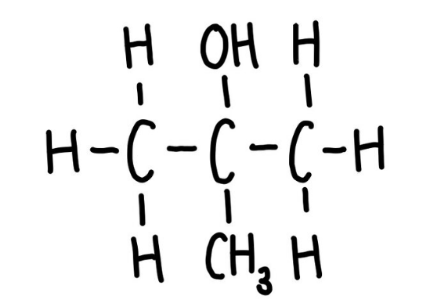
Tertiary alcohol
Three carbons bonded to the functional group carbon.
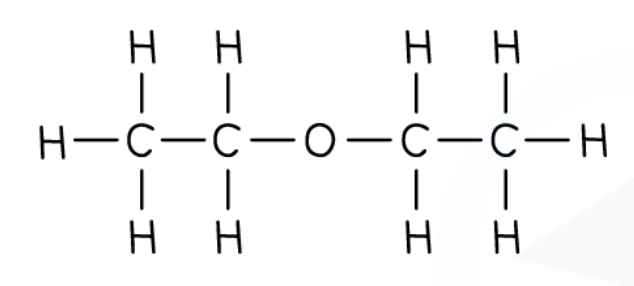
Ethers
General formula: CnH2n + 2O
Functional group: R-O-R
The alkoxy group
R represents another alkyl group.
Nomenclature: alkoxy alkane
Ethers have the same molecular formula as alcohols, but a completely different arrangement.
These are called isomers.
The Carbonyl Compounds
Carbonyl compounds contain the C=O functional group.
General formula: CnH2nCHO.

Aldehydes
Functional group: RCHO
Nomenclature: alkan + al
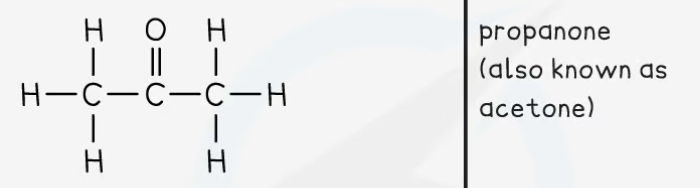
Ketones
Functional group: RCOR
Nomenclature: alkan + one

Carboxylic acids
General formula: CnH2n + 1COOH
Functional group: RCOOH
Nomenclature: alkan + oic acid
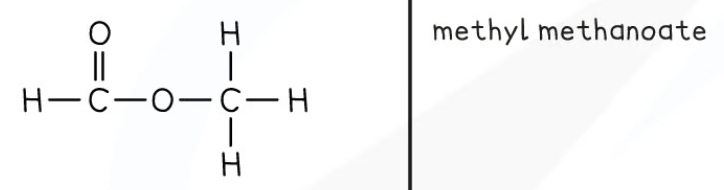
Esters
Functional group: RCOOR
Called the carboxylate group.
Nomenclature: alkyl alkanoate
The alkyl group is attached to the oxygen.
Esters are isomers of their fellow carboxylic acid.
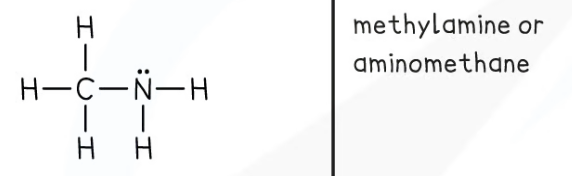
Amines
General formula: CnH2n+1NH2
Functional group: -NH2
The amino group
This can also be -NH- if a secondary amine.
Nomenclature: alkyl amine
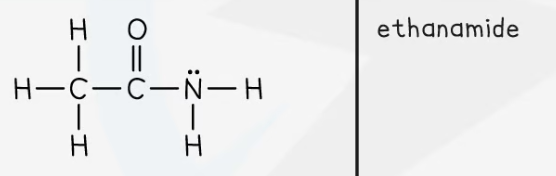
Amides
General formula: CnH2n+1CONH2
Functional group: -CONH2
The amido group
This can also be -CONH- if a secondary amide
Nomenclature: alkyl amide
Arenes
Arenes are compounds that contain rings.
In the ring, the π electrons are delocalised and move throughout the ring structure.
The ring is called a benzene ring.
These compounds are called aromatic compounds.