Enthalpy
1/27
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What is thermochemistry?
The study of heat changes during chemical reactions
What happens when a chemical reaction takes place?
Chemical bonds break and new ones are formed
What happens to the energy when bonds are broken and formed?
Energy is put in when bonds are broken and is given out when bonds are formed
What are exothermic reactions?
Reactions which give out energy to their surroundings
What is an example of an exothermic reaction?
Neutralising an acid with an alkali
What is an endothermic reaction?
A reaction which takes in energy from their surroundings
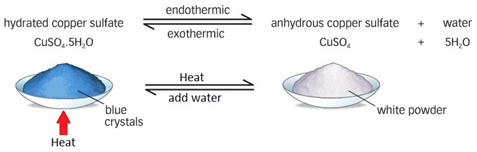
What is an example of an endothermic reaction?
Heating copper sulfate
Is bond breaking endothermic or exothermic and why?
Endothermic because energy is needed to break bonds
Is bond forming endothermic of exothermic and why?
Exothermic because energy is released when bonds are formed
What is bond enthalpy?
The energy needed to break a bond
What is the energy required to break a bond dependent on?
Where the bond is positioned - eg. it takes different amounts of energy to break the C—C bond in methane, ethane and ethene
What specific bond enthalpy do you use in calculations to take into account the varying bond enthalpies?
Mean bond enthalpy
What is an enthalpy change?
An energy change measured at constant pressure and under stated conditions
What are the units of enthalpy change, ΔH?
kJ mol⁻¹
What is the symbol for enthalpy?
H
Symbol for enthalpy change
ΔH⦵ - delta H under standard conditions
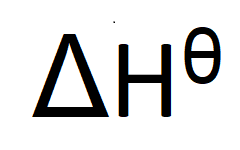
What does this symbol, ⦵, tell you about the reaction?
That the reactants and products were in their standard states and the measurements were made under standard conditions
What is the standard state of an element?
The state in which it exists at 298K and 100kPa
What are the standard conditions for measuring enthalpy changes?
100kPa (about 1 atm) pressure,
a stated temperature - normally room temp. of 298K (25°C) - eg. ∆H⦵₂₉₈
What are enthapy level diagrams used for?
To represent enthalpy changes
Enthalpy diagram for an exothermic reaction
Reactants are higher than the products
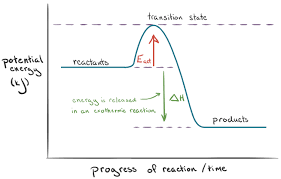
Why are the enthalpy changes for exothermic reactions negative?
The products end up with less heat energy than the starting materials as they lose heat energy when heating up their surroundings
Enthalpy diagram for an endothermic reaction
Reactants are lower than the products
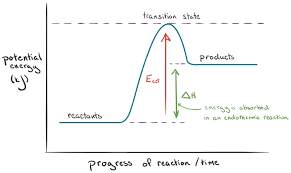
Why are the enthalpy changes of endothermic reactions positive?
The products end up with more energy than the starting materials as it takes in heat from the surroundings
How does pressure affect the amount of heat energy given out by reactions that involve gases?
If a gas is given out, some energy is required to push away the atmosphere. The greater the atmospheric pressure, the more energy is used for this.
What can also affect the enthalpy change of a reaction?
Physical states
Two possible outcomes for hydrogen burning in oxygen to form water:
H2 (g) + ½ O2 (g) → H2O (l)
ΔH = -285.8 kJ mol-1
H2 (g) + ½ O2 (g) → H2O (g)
ΔH = -241.8 kJ mol-1
What does the difference in ΔH between forming liquid water and steam represent?
The amount of heat needed to turn one mole of water into stream