lecture 11 - ceramics, glasses, and glass-ceramics
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
are ceramics/glasses organic or inorganic
inorganic
are ceramics/glasses metallic or non-metallic
non-metallic
what are some uses for ceramics/glasses
eyeglasses
diagnostic instruments
drug carriers
what are ceramics mostly used to repair/replace
skeletal hard connective tissue
what 2 things do ceramic implant success depend on
stable attachment to connective tissue (bulk implants)
ability to stimulate repair and regenerate bone (particulates for bone grafting)
what are the 4 types of bioceramics
type 1 - inert and dense
type 2 - nearly inert, porous
type 3 - bioactive
type 4 - resorbable
what happens if a biomaterial is toxic
tissue dies
what happens if a biomaterial is non-toxic and bioinert
a fibrous capsule forms
what happens if a biomaterial is non-toxic and bioactive
native tissue/cells infiltrate the biomaterial
what happens if a biomaterial is non-toxic, bioactive, and degradable
native tissue replaces the biomaterial over time
most ceramics are biomaterials that are ____ and ____
non-toxic and bioactive (type 3)
what is an interfacial bond
the bond between ceramic and bone tissue
what does the strength of the interfacial bond depend on
how bioactive the material is
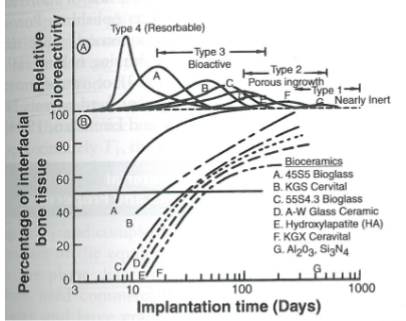
what does this graph show
% of interfacial bone tissue as a function of implantation time
how are type 1 ceramics implanted
through morphological fixation (like aluminum)
what do type 1 ceramics exhibit with a perfect fit
very thin fibrous capsule
how are type 2 ceramics implanted
through bone growing into the pores of the ceramic
type 2 ceramics can withstand ___ complex stress than type 1
more
because they are porous type 2 ceramics have ____ overall strength than type 1
lower
pore size should be how large to be conducive to bone ingrowth
>100microns → allows for cells and blood vessels to fit within pores to keep cells alive
type 3 ceramics are ___-reactive
surface
how are type 3 ceramics implanted
by chemically bonding directly with bone
how are type 4 ceramics implanted
embedded within bone to be slowly replaced by bone
because type 4 ceramics degrade, what 2 rates have to match
rate of degradation and rate of tissue formation
what does the density of a ceramic depend on
thermal processing (how high the temp is and how long it is kept at a certain temp for)
how are porous ceramics made
by adding a second phase that decomposes before densification (leaves behind pores)
what is sintering
a process that uses temp and time to help particles fuse
ceramics are also known as what
oxides
what is an example of a nearly inert crystalline ceramic
alumina (Al2O3)
why are alumina excellent for corrosion resistance
because they are already oxidized
what are 4 pros of alumina
good compatibility
high wear resistance
high wettability
low coefficient of friction
why is alpha-alumina used as a biomaterial
because the alpha form is the most thermodynamically stable