lec 28 - autoimmunity and tolerance
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is self tolerance
a state in which an individuals immune system does not attack the normal tissues of the body
what is autoimmunity
a breakdown or failure of the mechanisms of self-tolerance
how we get self tolerance
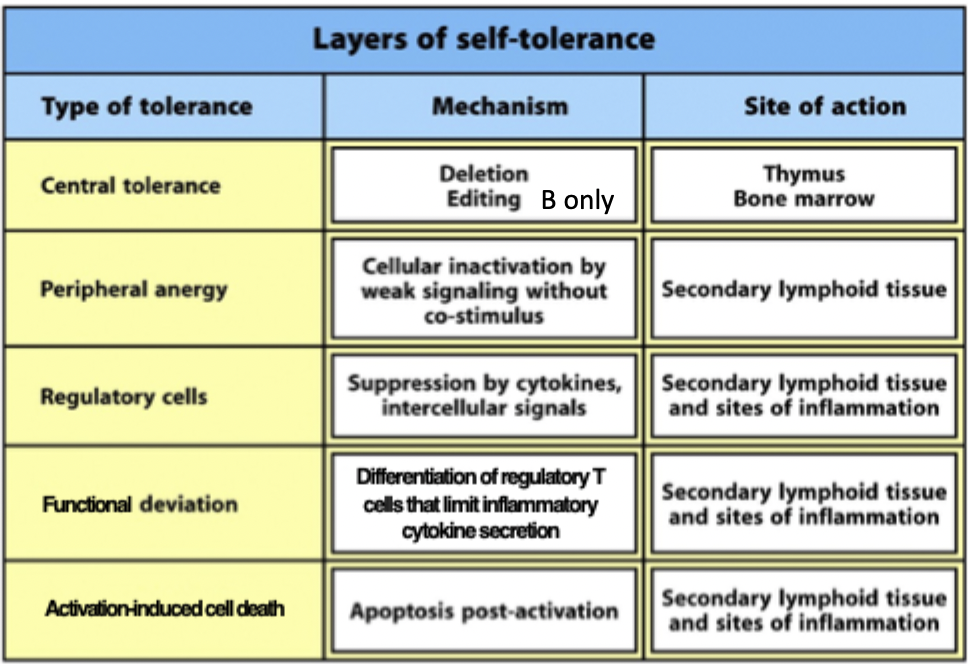
during lymphocyte development potentially self-reactive T and B cells are identified and deleted - central tolerance
some self-reactive cells escape elimination
tolerance induced in mature lymphocytes that have left the central lymphoid organs is peripheral tolerance
central tolerance - B cells development
initial testing of the BCR on immature B cells
no self reactivity - migrates to the periphery and matures
self reactivity - negative selection
self reactive B cells what happens
anergy (unresponsiveness or paralysis of function)
elimination/deletion (apoptosis)
rescue by receptor editing (continued expression of RAG which can allow cells to continue light chain rearrangement leading to alteration of specificity
central tolerance - T cell development
dangerously autoreactive DP thymocytes (i.e. strongly reactive with self MHC or MHC with self peptide) are removed by negative selection
cells strongly reactive with MHC presenting self antigen are also removed
AIRE creates a peripheral shadow i the thymus - expresses small amounts of tissue specfic proteins to test against T cell receptors
AIRE
a transcription factor that causes a small amount of lots of different proteins in the thymus so that T cells can
peripheral tolerance - what
occurs after central tolerance when mature B and T cells have migrated into the periphery
mechanisms of peripheral tolerance
anergy (unresponsiveness)
Tregs
functional deviation (autoreactive T cells → induced Tregs)
activation induced cell death - hyperactivity of cells can lead to them dying or anergy
layers of self tolerance diagram
how to induce peripheral tolerance
very large doses of antigen
regulatory T cells
oral antigen - because GIT designed to not react to oral antigen
types of autoimmunity
breakdown of central tolerance - APECED
breakdown of peripheral tolerance - Treg function - IPEX
breakdown of peripheral tolerance (self antigen) - multiple sclerosis
APECED - what symptoms
highly variable disease phenotype
failure of multiple endocrine glands
chronic mucocutaneous candadiasis
ectodermal symptoms (e.g. pitted nail dystrophy, dental enamel hypoplasia, alopecia, vitiligo)
APECED - disease mechanism
individual organs of the body express tissue-specific antigens
in the thymus T cells arise capable of recognising tissue-specific antigens
under control of the AIRE protein thymic meduallary cells express tissue-sepcific proteins, deleting tissue-reactive T cells
but in the absence of AIRE, T cells reactive to tissue-specific antigens mature and leave the thymus - not exposed
how does APECED lead to symptoms
autoimmune reactions to endocrine glands leads to type I diabetes, addison’s disease, hypopituitarism etc
to self antigens leads to ectodermal symptoms eg enamel hypoplasia, alopecia
why are people with APECED susceptible to candadiasis
associated with autoantibodies against IL-17
Th17 cells are important for protection against fungi at the epithelium
IPEX
breakdown of peripheral tolerance
characterised by systemic autoimmunity, typically in 1st year of life
caused by mutation in FoxP3
multiple sclerosis
impaired peripheral
T cell mediated neurological disorder
destructive immune response against brain antigens eg myelin basic protein
symptoms include muscle weakness, blindness, paralysis
multiple sclerosis pathogenesis
unknown trigger sets up initial focus of inflammation in brain and blood-brain barrier becomes locally permeable to leukocytes and blood proteins
T cells specific for CNS antigen and activated in peripheral lymphoid tissues reencounter antigen presented on microglia or dendritic cells in brain
inflammatory reaction in brain due to mast-cell activation, complement activation, antibodies and cytokines
demyelation of neurons
MS treatment
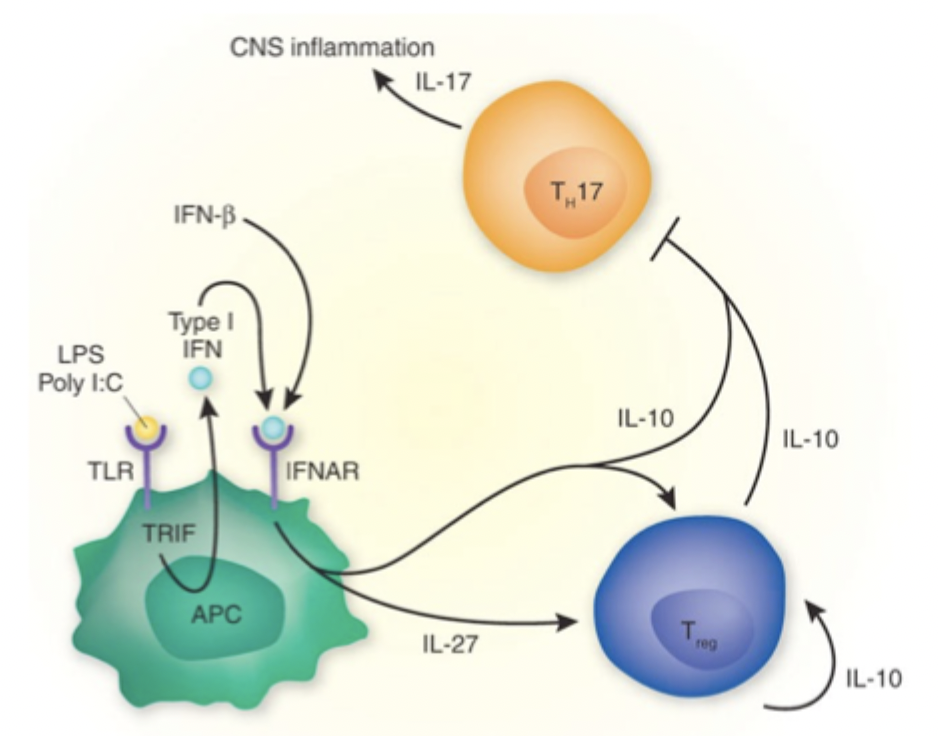
type I interferon
act on APCs to produce Tregs which can produce cytokines to reduce inflammation
caused by Th17
not curative
betaferon
fingolimod
derived from myriocin isolated from the fungus isaria sinclairii
prevents egress of T and B cells from secondary lymphoid organs, preventing cells from entering the blood and tissues (including the CNS)
types of risk factors for autoimmune disease
genetic factors
environmental factors
host factors
genetic risk factors for autoimmune disease
gene loci associated with susceptibility (often genes encoding molecules of the immune system)
epigeneitc changes (DNA methylation, histone modification) which alter gene accessibility
environmental risk factors for autoimmune disease
infection (bacterial or viral - e.g. epstein barr virus)
geographical - sunlight exposure?
also host factors - age, sex (F»M), ethnicity