CHLORIDE
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Chloride
Major extracellular anion which is the counterpart of Na+
Water, osmotic
Chloride Shift, Hamburger Phenomenon
Chloride
Functions:
_ balance and _ pressure (w/ Na+)
Regulates acid-base balance (_ _/_ _)
Hartog Jacob Hamburger
Chloride
Functions:
Who discovered the chloride shift?
bicarbonate, chloride
gastric juice
Chloride
Functions:
The exchange of _ and _ across the membrane of red blood cells (RBCs) in the cardiovascular system
Contributes to the acidity of _ _
Kidneys
GI tract
Sweat
Chloride
Excretions (3)
Hypochloremia
Chloride
Decreased concentration of chloride ions in the blood
Hyperchloremia
Chloride
Increased concentration of chloride ions in the blood
Prolonged vomiting
Diabetic ketoacidosis
Aldosterone deficiency
Salt losing renal diseases such as pyelonephritis
Metabolic alkalosis
Profuse sweating
Addison’s disease (low cortisol and aldosterone)
Hypochloremia Causes (7)
Dehydration
GI losses
Renal Tubular Acidosis (RTA)
Metabolic acidosis
Congestive heart failure
Cushing’s syndrome
Hyperchloremia Causes (6)
Serum
Plasma (lithium/ammonium heparin)
24 hour urine
Sweat
Specimen (4)
lower, HCO3-
decreased
Interferences
Slightly _ in postprandial specimen serum with high concentration of _
Hemolysis - false _
ISE method
Methods of Measurement
The most common method
ISE method
Methods of Measurement
Uses silver sulfide and silver chloride membrane
Amperometric-coulometric
Methods of Measurement
Accurate
Gives direct read-out chloride concentration
Cotlove
chloridometer
Methods of Measurement
Amperometric-coulometric
Adaptations: _ (digital) titrator or _
Schales and Schales
Methods of Measurement
Cl ions + Mercuric nitrate to form mercuric chloride (soluble) with drop of mercuric nitrate, the indicator will change from colorless or faint pink to violet
faint pink to violet
Methods of Measurement
Schales and Schales color reaction
Zall color reaction
Methods of Measurement
Reagent contains Mercuric thiocyanate and ferric nitrate
Reddish brown ferric thiocyanate
Methods of Measurement
Zall color reaction
Schoenfeld & Lewellen
Methods of Measurement
Method used in the experiment
mercuric chloride
ferric ions
orange-yellow
480-492
Methods of Measurement
Schoenfeld & Lewellen
Principle: Chloride ions in serum displaces thiocyanate ions from the mercuric thiocyanate forming _ _ which is only very slightly ionized. The liberated thiocyanate ions react with _ _ to form an _-_ thiocyanate complex which is measured at _-_ nm. The color intensity is proportional to the chloride concentration
492
Methods of Measurement
Schoenfeld & Lewellen
Mix well and read at _ nm against water blank after one minute
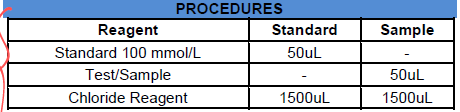
100 mmol/L
Computation factor
1
Conversion factor
mEq/L
SI unit
96-110 mmol/L
Normal Range
Serum
125-135 mmol/L
Normal Range
CSF
170-255 mmol/L
Normal Range
Urine (24H urine sample)
TPTZ Method, Photometric Colorimetric Test
Method of Measurement (lab manual)
blue colored complex
Method of Measurement (lab manual)
Color reaction
355
Computation factor if mg/dL