Chemistry : Chemical equilibria AS level
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
reversible reactions
products can react to reform the original reactants
dynamic equilibrium
a state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in constant concentrations of products and reactants.
A closed system
in which no matter is exchanged with the surroundings, allowing equilibrium to be established.
Conditions for equlibrium of gases
equilibrium can onlybe reached in a closed system
Le Chatelier's Principle
states that if a dynamic equilibrium is disturbed, the system will adjust to counteract the change and restore equilibrium.
position of the equilibrium
refers to the relative amounts of reactants and products at equilibrium, which can be influenced by changes in concentration, temperature, and pressure.
Increase in concentration of a reactant
Equilibrium shifts to the right , (reduce the effect of an increase in the concentration of a reactant)
Decrease in concentration of a reactant
Equilibrium shifts to the left
Effects of pressure
Changes in pressure only affect reactions where the reactants or products are gases
Increase in pressure causes the equilibrium to shift towards the side with fewer moles of gas, reducing the overall pressure.
Decrease in pressure causes the equilibrium to shift towards the side with more moles of gas, increasing the overall pressure.
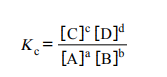
equilibrium expression
Expression that links equilibrium constant to the concentrations of reactants and products at equilibrium. Kc only changes if the temperature of reaction changes
Solids are ignored in equilibrium expressions
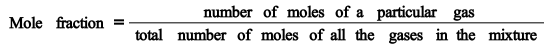
Mole fraction
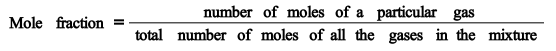
Partial pressure
equals mole fraction times the total pressure
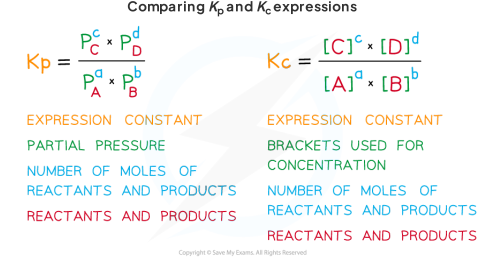
Comparing Kc and Kp
Kc is the eqilibrium constant based on concentrations, while Kp is based on the partial pressure of gases
Acids
substances that release hydrogen ions when theydissolve in water
Acid + Base is a neutralization reaction. It forms salt and water
Base
a substance that accepts hydrogen ions or a compound that contains oxide or hydroxide ions.
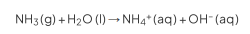
The Brønsted-Lowry Theory
defines acids and bases in terms of proton transfer between chemical compounds
The Brønsted-Lowry Theory Acid/Base
species that gives away a proton (for acids )
species that accepts a proton using its lone pair of electrons (for bases)
GK
Species that can act both as acids and bases are called amphoteric
Strong Acids
an acid that dissociates almost completely in aqueous solutions. F.E: HCl, nitric acid, sulfuric acid
Electronegativity
is the ability of an atom to attract a a pair of electrons towards itself in a covalent bond
Pauling scale
used to assign the value of e;lectronegativity
What happens when there is an increase in no.of protons
causes the nuclear attraction to increase as well as increasing electronegativity - therefore larger nuclear attraction which bonds the electrons more strongly
atomic radius
is the distance between the nucleus and the electrons in the outer most shell
Sheilding effect
grearter the no.of shells, less the outer electron is attracted to it s nucleus
trends in electronegativity - down a group
Going down the group there is a decrease on electronegativity - as sheilding and atomic radius increase down a group
trends in electronegativity - across a period
across a period electronegativity increases - nuclear charge across a period increases whereas the shielding remains the same. Atomic radius decreases
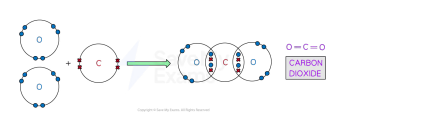
Covalent bonds
are formed by sharing a pair of electrons between two atoms
occurs between 2 non-metals
covalent compounds are crystalline lattice
Diatomic molecules
have equal distribution of bond pair leads to non polar molecule
laws of electronegativity
the least electronegative atom’s electron will transfer to the other atom - leading to an ionic bond
Ionic bond
involves the transfer of electrons froma metal to a non metal leaving it with a full outer shell
Electrostatic attractions
are formed between the oppositely charged ions to form ionic compounds - this form of attraction is very strong and requires a lot of energy to overcome
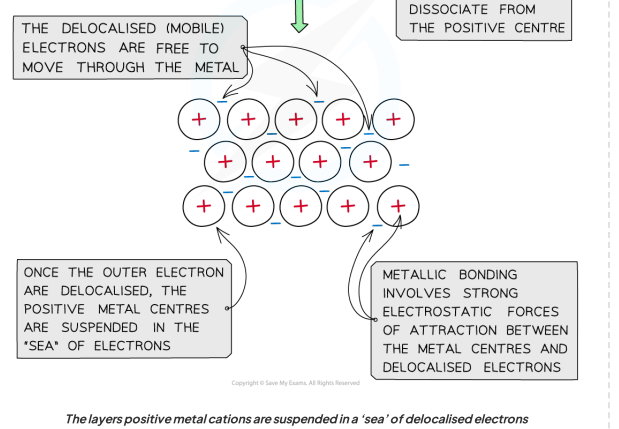
How are atoms affected by the delocalised electrons ?
Metal atoms become positively charged
double covalent bonding
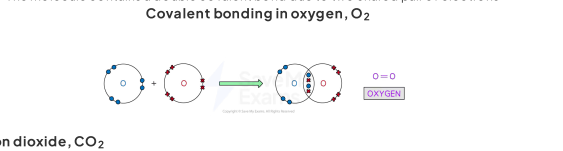
triple covaent bonding
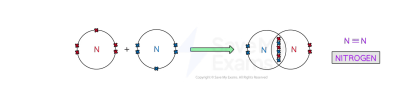
dative covalent bonding or coordinate bonding
iswhen the both electrons are from the same atom - for example
Ammonium ion
Aluminum chloride
Pressure
volume is inversley proportional to the pressure
temparature is directly proportional to pressure
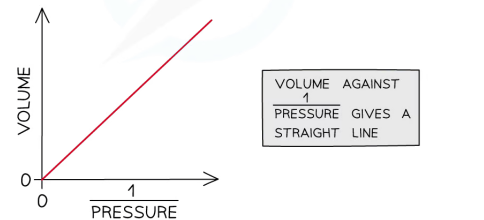
Kinetic theory of gases
gas molecules move very fast and randomly
hardly have any volume
gas molecules do not attract or repel each other
have elastic collisions
Ideal gases
Ideal gases depend on its
pressure
temperature
Volume is directly proportional to the temperature
Limitations of ideal gas law
Ideal gases do not obey the kinetic theory of gases at very high or low temperatures, because
molecules are close to each other
volume of gases isn’t negligible
pd-pd forces between the molecules
attractive forces pull molecules away from container walls
ideal gas equation
pV = nRT
p = pressure in pascals
R = gas constant (8.31 j/k mol
n = number of moles of gas
T = temperature in kelvin (K)
Giant Ionic lattices
Type of lattice formed depends on the sizes of the anion and the cation. For example:
MgO and NaCl are cubic
Covalent lattices
Covalent compunds can either be arranged in simple molecular or giant molecular
Example sof simple molecular:
Iodine, Ice, ,buckminsterfullerene
Examples of giant molecular:
graphite, diamond and silicon(IV) oxide
giant metalllic lattices
in which metal ions are surrounded by a sea of delocalised electrons
often packed in hexagonal layers or in a cubic arrangement
Physical Properties of Ionic bonding & giant ionic lattice structures
Ionic compunds are strong
They are brittle
Have high melting and boiling points (depends on its charge density)
soluble in water
can form ion-dipole bonds
conduct electricity when molten or in solution
Metallic bonding & giant metallic lattice structures
Metallic compunds are malleable
strong and hard
high melting and boiling points
Pure metals are insoluble in water
conduct electricity while in solid or liquid state
Covalent bonding and simple covalent lattice
have low melting and boiling points
most compounds are insoluble in water
do not conduct electricity
Covalent bonding & giant covalent lattice structures
high melting and boiling points
insoluble in water
only graphite conducts electricity
can be hard or soft (graphite soft, diamond hard)
Standard enthalpy change of reaction(endo and exo)
The enthalpy change when the reactants in the stoichiometric equation react to form the products under standard conditions
Standard enthalpy change of formation(endo and exo)
The enthalpy change when 1 mole of compound is formd from its elements USC
Standard enthalpy change of combustion(exo)
The enthaply change when one mole of substance is burnt in excess oxygen USC
Standard enethalpy change of neutralisation(exo)
The enthalpy change when one mole of water is formed by reacting and acid and an alkali USC
Exothermic reactions
Heat is given off by the reaction to the surrounding
products have less energy than the reactants
exothermic reactions are thermodynamically possible
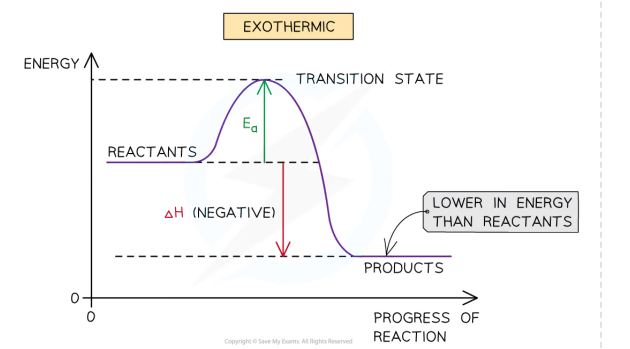
Endothermic reactions
Heat is absorbed by the system from the surroundings
Enthalpy change of endo is always positive
products have more energy than the reactants
reaction pathway diagrams
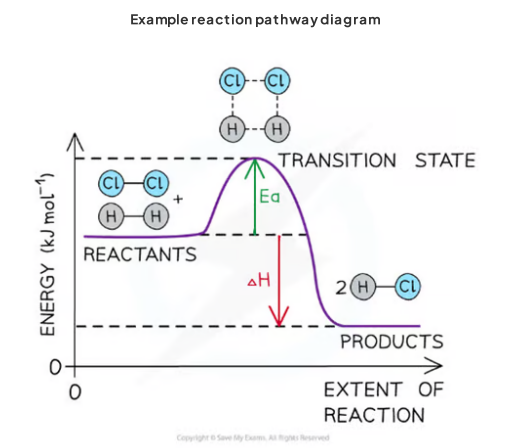
activation energy (Ea)
The minimum energy vthat colliding particles must have for a collision to be successful and a reaction to take place
standard conditions
pressure - 101kPa
temperature - 298K
Bond breaking
Energy need to overcome the attractive forces so its endothermic
Bond forming
Energy is released from the reaction to the surroundings when new bonds are formed, therefore its exothermic
Calorimetry
a technique used to measure changes in enthalpy of chemical reactions
specic heat capacity
The energy needed to increase the temperature of 1g of a substance by 1 degree celcius
SHC - equation
q = mcT
q = heat transferred, J
m = mass of the water, g
c = specific heat capacity
T = temperature change
Rate of reaction
the speed at which a chemical reaction takes place

Collision theory
For a chemical reaction to occur
the particles must collide with each other in the correct orientation
must colide with enough energy
Collision frequeny
number of collisions per unit time