12.2: MIxtures and Solutions
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
Pure Substance
Characterized by an unchanging or specific composition
Are substances that are made up of only one kind of particle and have a fixed or constant structure
As pure substances and mixtures
How can matter be classified?
Elements
The simplest form of matter that is composed of only one kind of atom
A substance that results from a combination of only one type or kind of atom
Cannot be broken down into simpler substances
Compounds
Composed of two or more elements combined chemically in definite proportions
A substance that results from a combination of two or more difference chemical elements
Mixture
Composed of different pure substances that are physically combined in variable proportions
Physical combination of two or more substances that blend together without forming new substances
Heterogeneous Mixture
A mixture that contains distinct substances that are physically and often visibly-separate
Not evenly distributed in the sample
Homogeneous mixture
A mixture that has the same uniform appearance and composition all throughout
Exist in a single phase, this means that the appearance, properties, and composition are uniform through a sample
Solution
Can be physically combined in varying proportions
Solute
Substance that gets dissolved (smaller amount than the solvent)
Dissolving
The one that does the dissolving
Alloy
Metal made by combining two or more metallic elements
Suspension
The solid particles are spread throughout the liquid without dissolving in it

Colloid
Particles of an insoluble substance are suspended throughout another substance
Mixture
Identify if it is an element, mixture or compound:
Air
MIxture
Identify if it is an element, mixture or compound:
Brass
Mixture
Identify if it is an element, mixture or compound:
Bronze
Compound
Identify if it is an element, mixture or compound:
Water
Element
Identify if it is an element, mixture or compound:
Graphite
Coarse mixture
Identify what kind of heterogenous mixture:
Halo-halo

Colloid
Identify what kind of heterogenous mixture:
Toothpaste

Colloid
Identify what kind of heterogenous mixture:
Muddy water

Colloid
Identify what kind of heterogenous mixture:
Whipped cream

Homogeneous mixture
Is Solution a heterogenous or homogeneous mixture?
Heterogeneous mixture
Is Suspension a heterogenous or homogeneous mixture?
Heterogeneous mixture
Is Colloid a heterogenous or homogeneous mixture?
O2
Identify the solute:
O2 - 21%
N2 - 78%
(Air)
N2
Identify the solvent:
O2 - 21%
N2 - 78%
(Air)
Aqueous solution
A solution in which water is the solvent
Filtration
1?
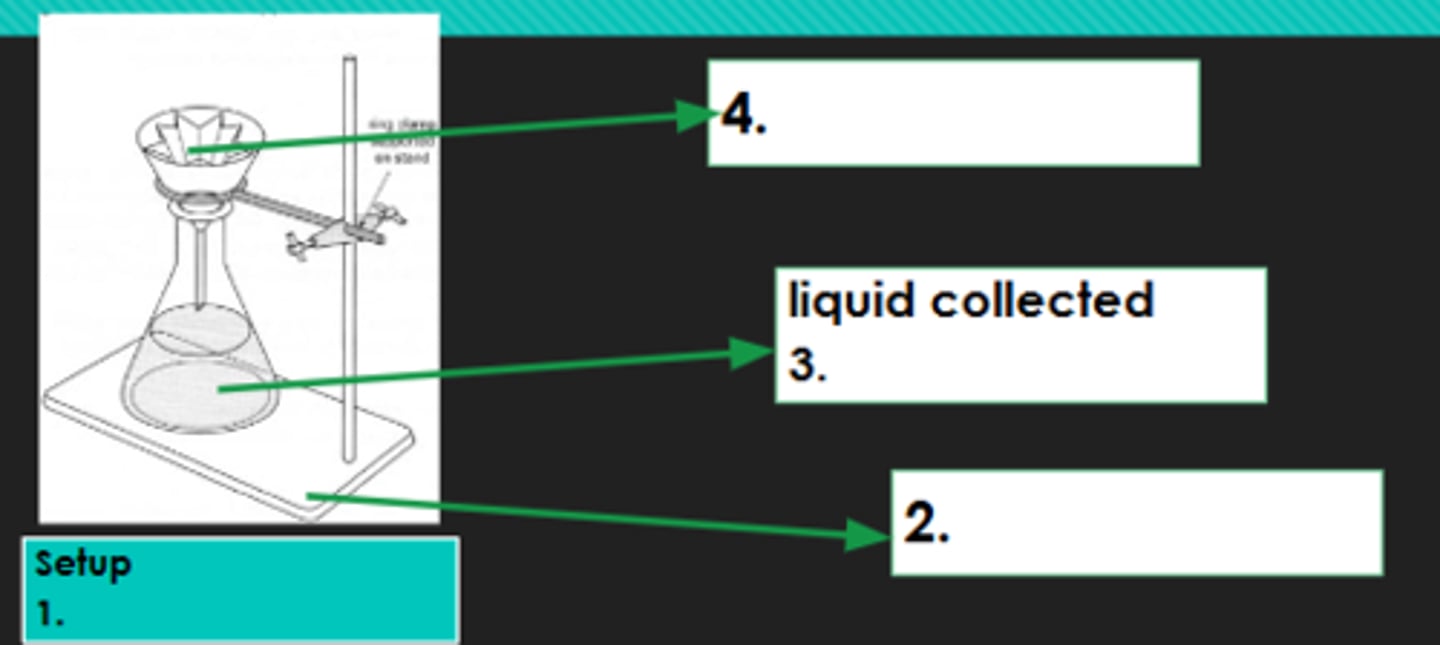
Iron stand
2?
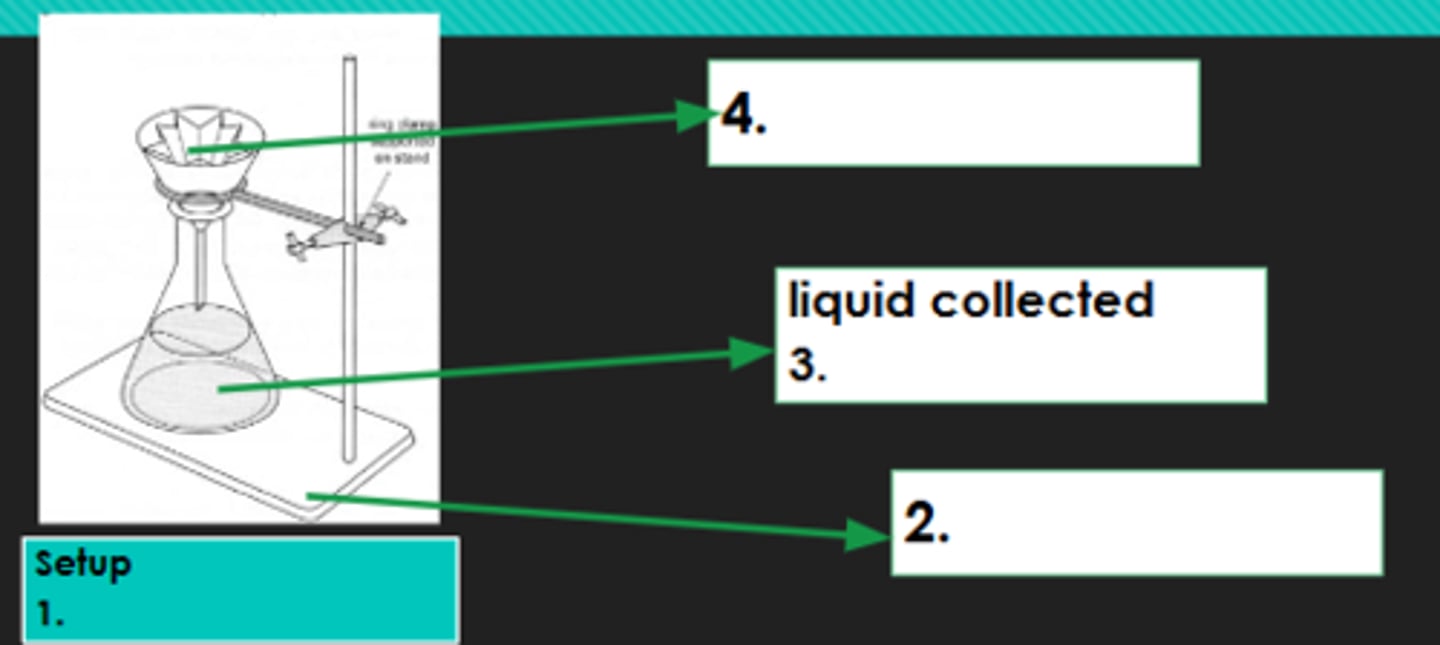
Filtrate
3?
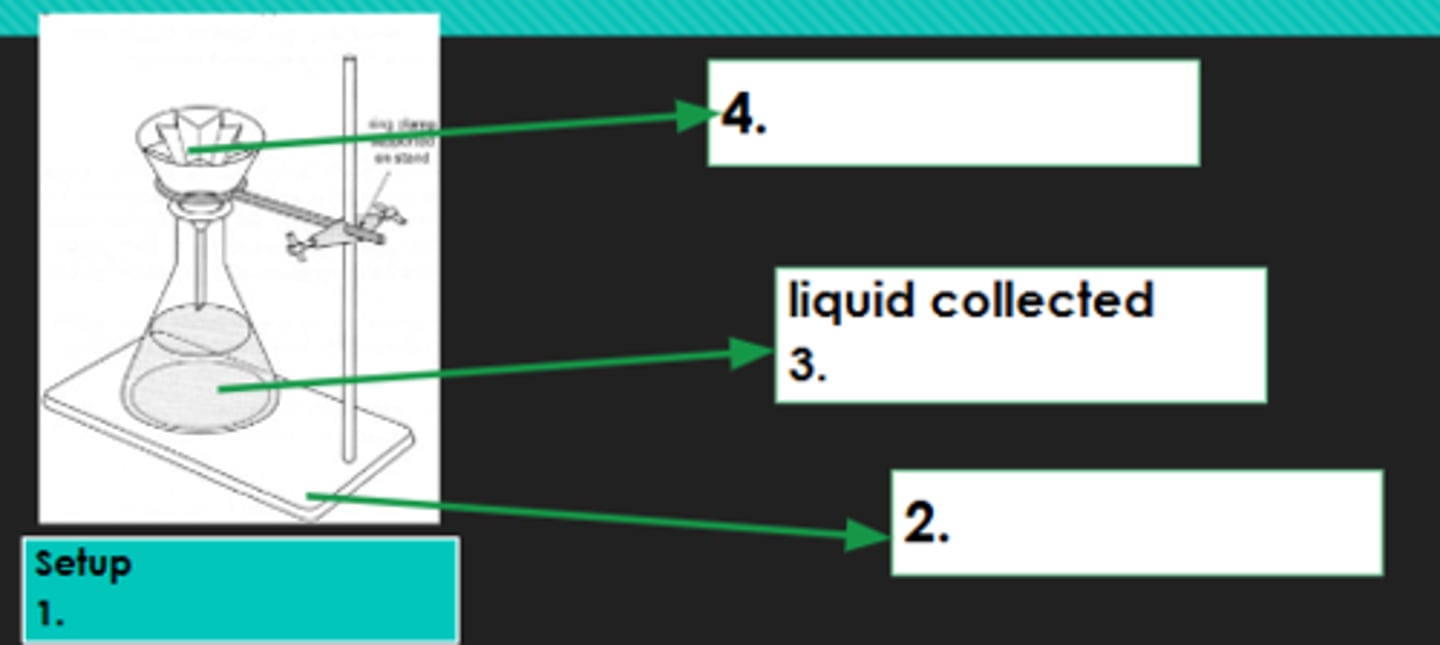
Filter paper
4?
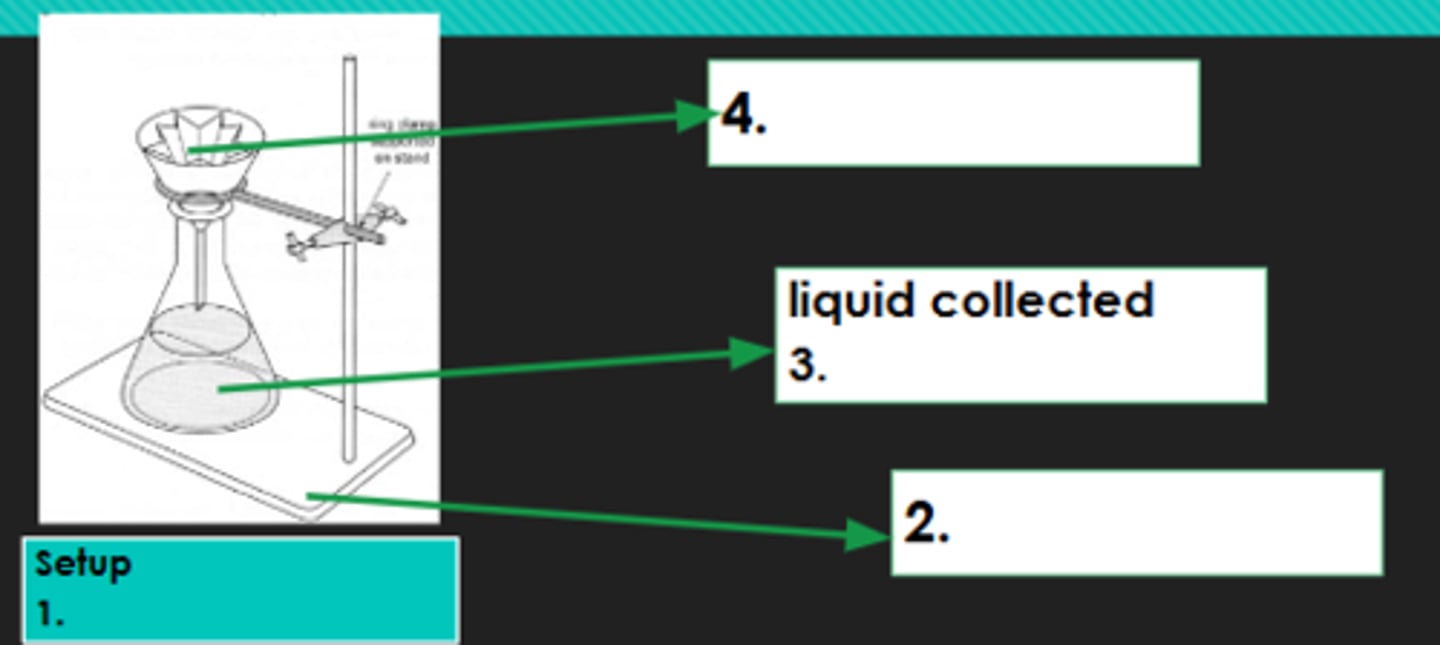
Evaporation
5?
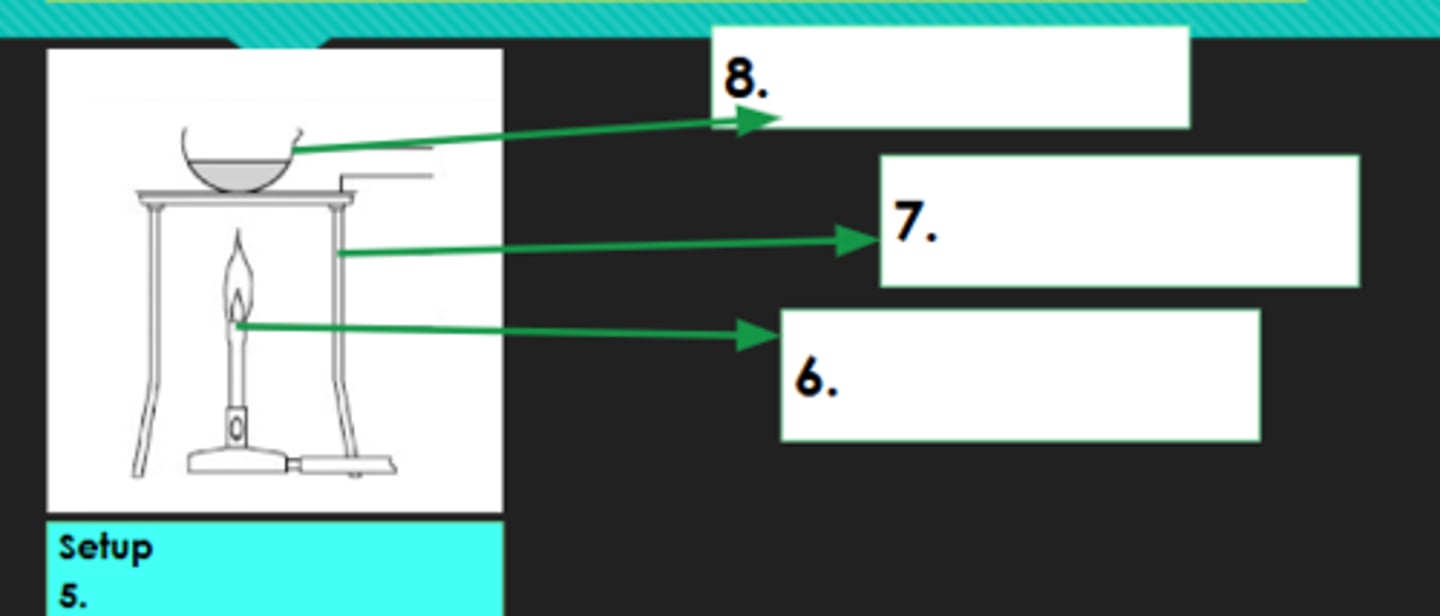
Bunsen burner
6?
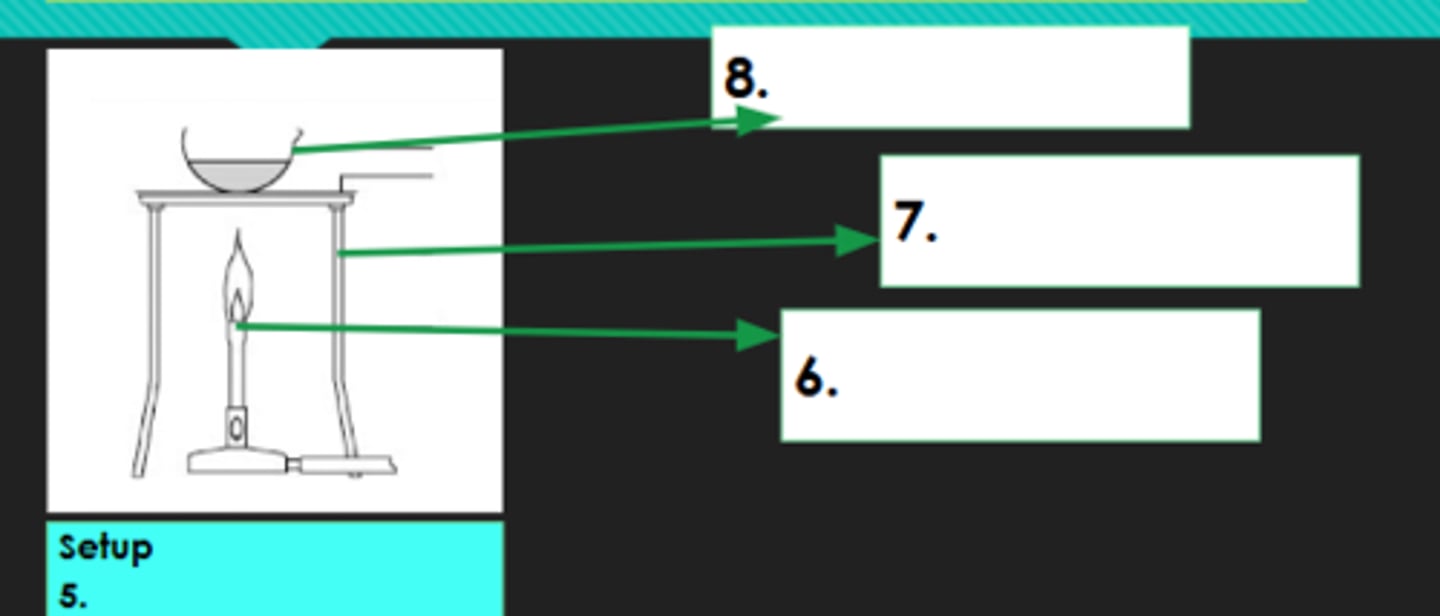
Tripod
7?
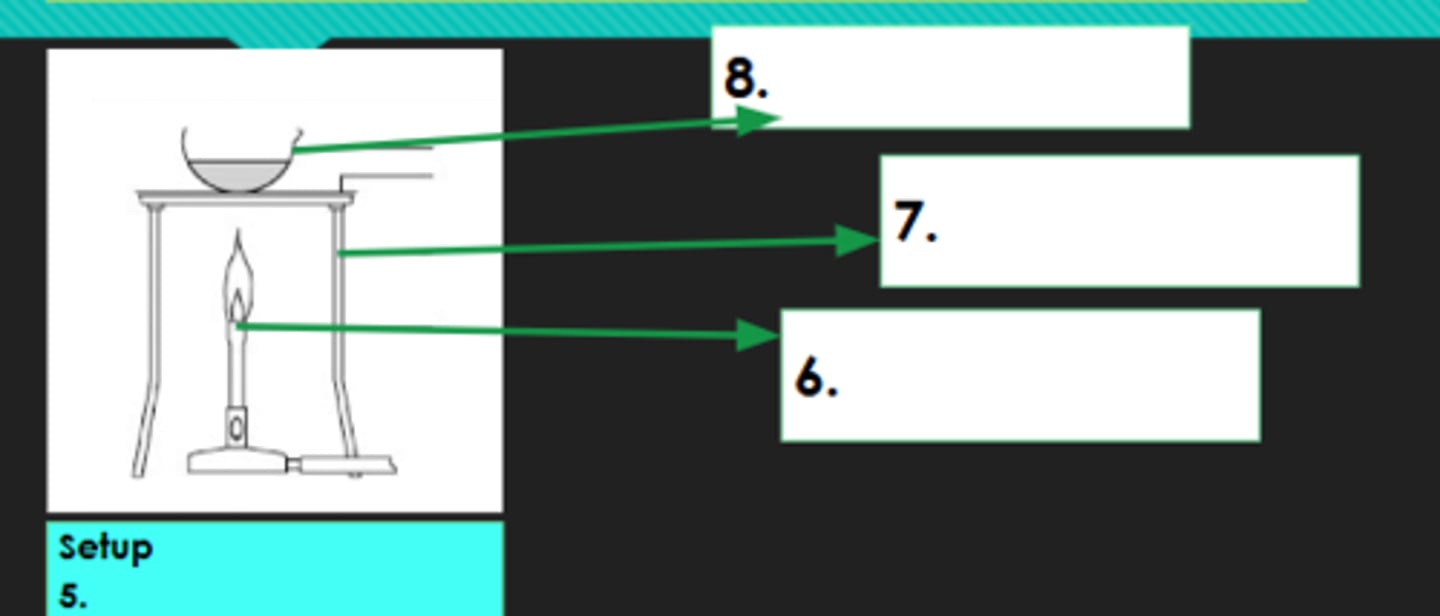
Evaporating dish
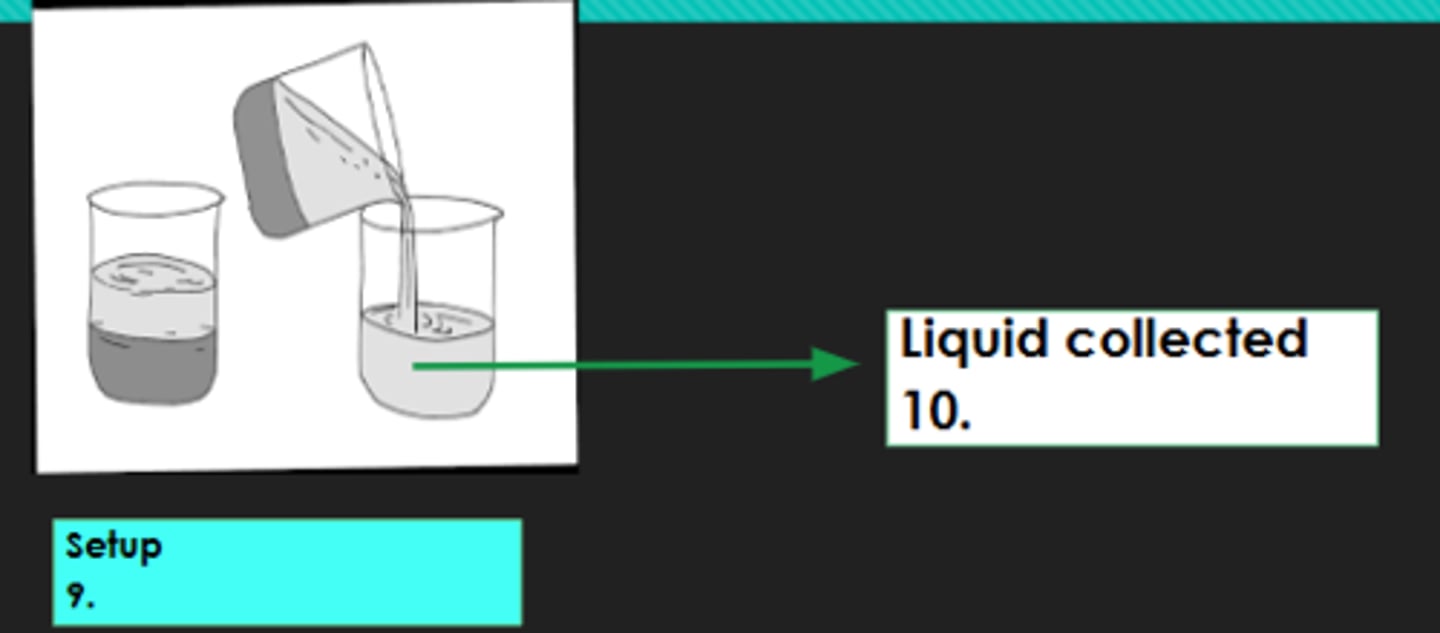
8?
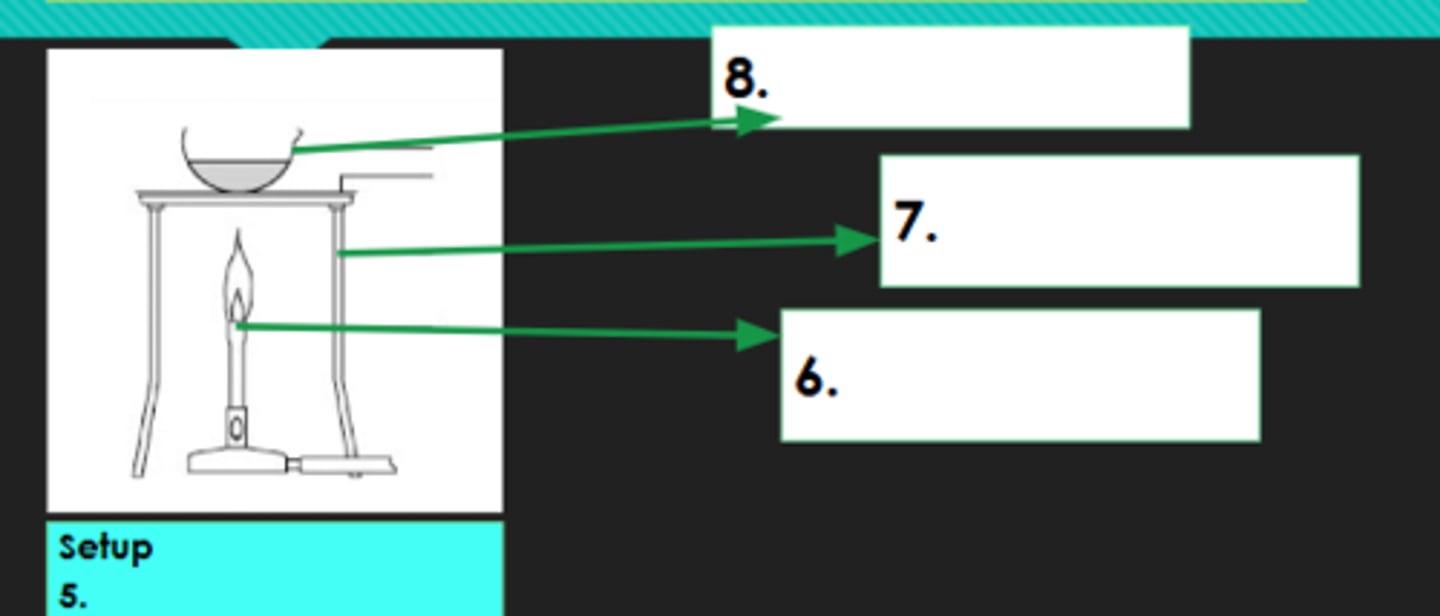
Decantation
9?
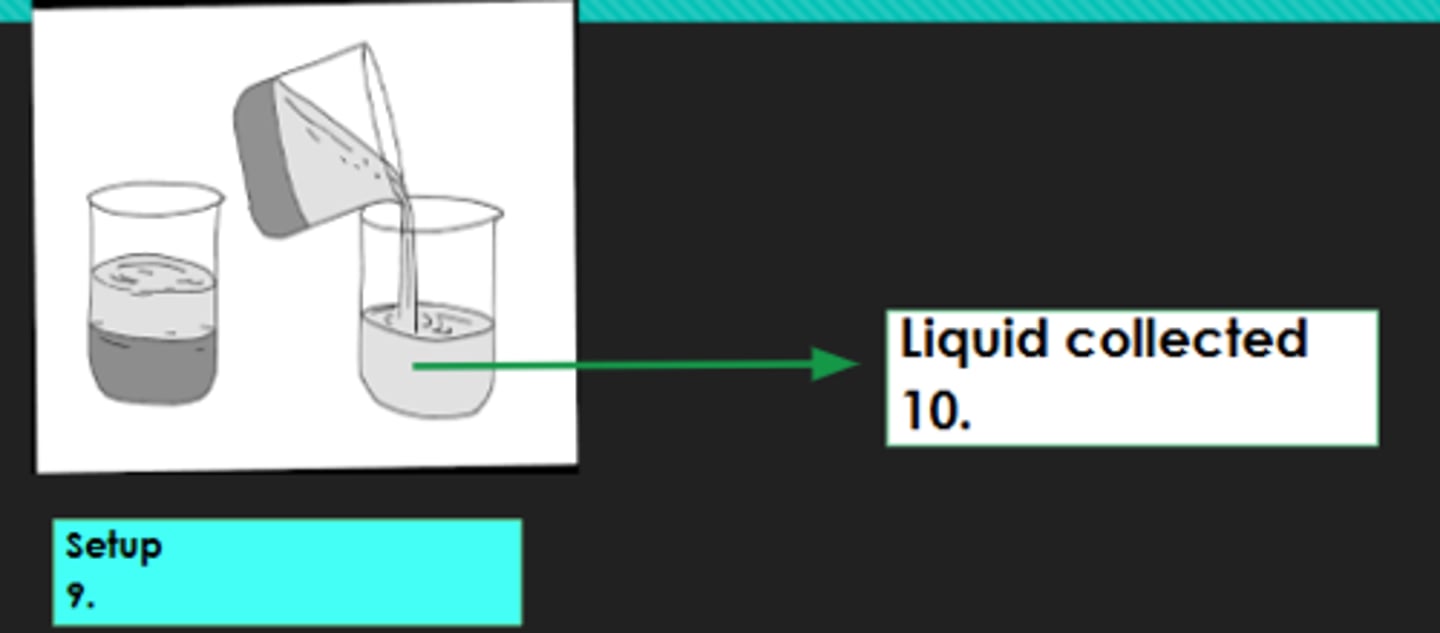
Decantate
10?
Distillation
11?
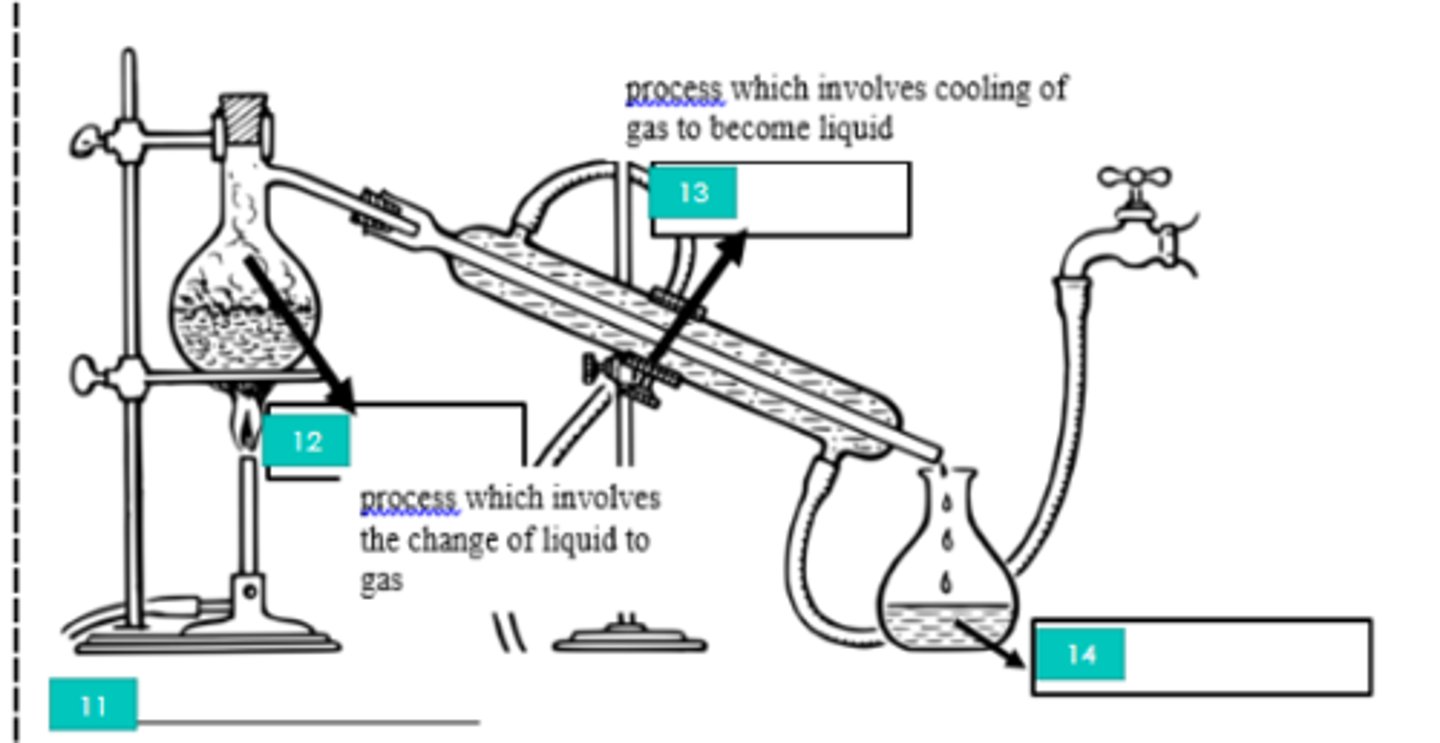
Evaporation
12?
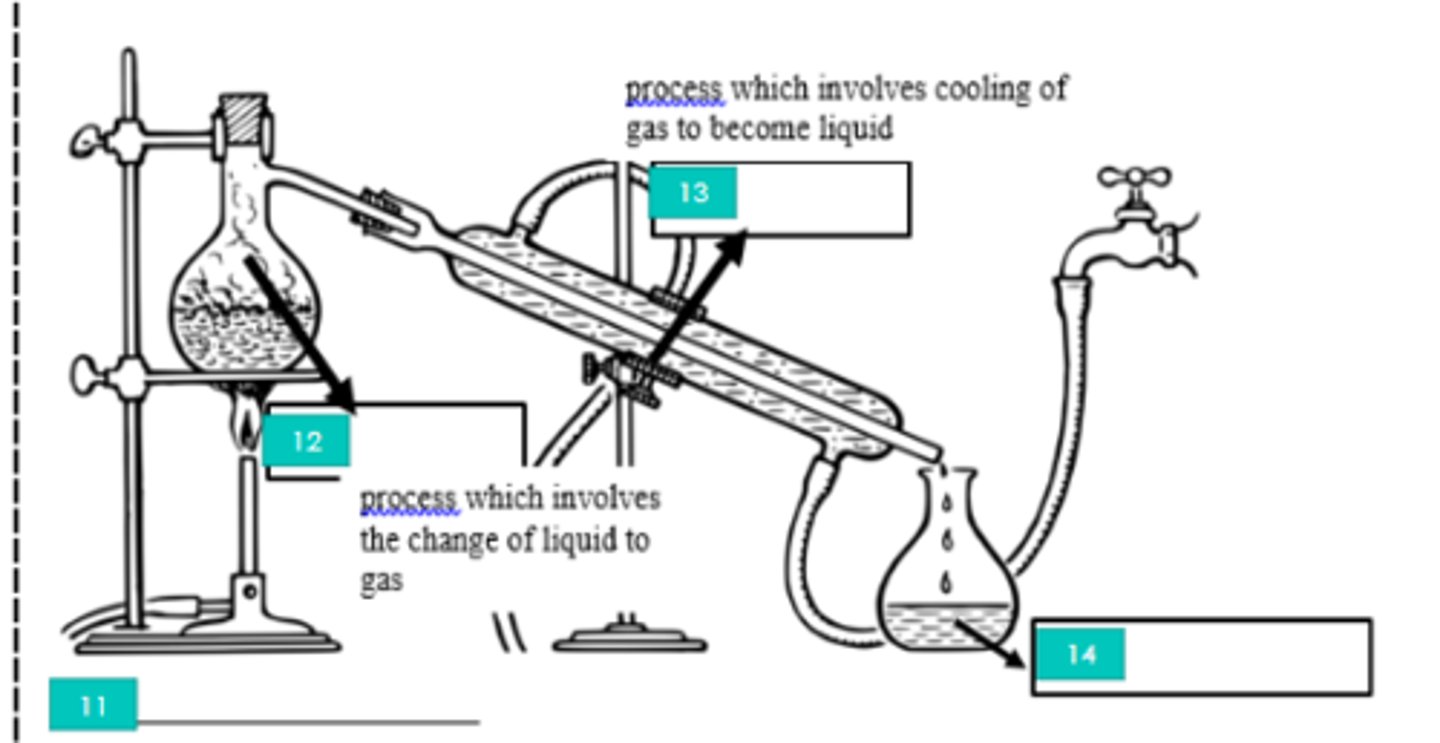
Condensation
13?
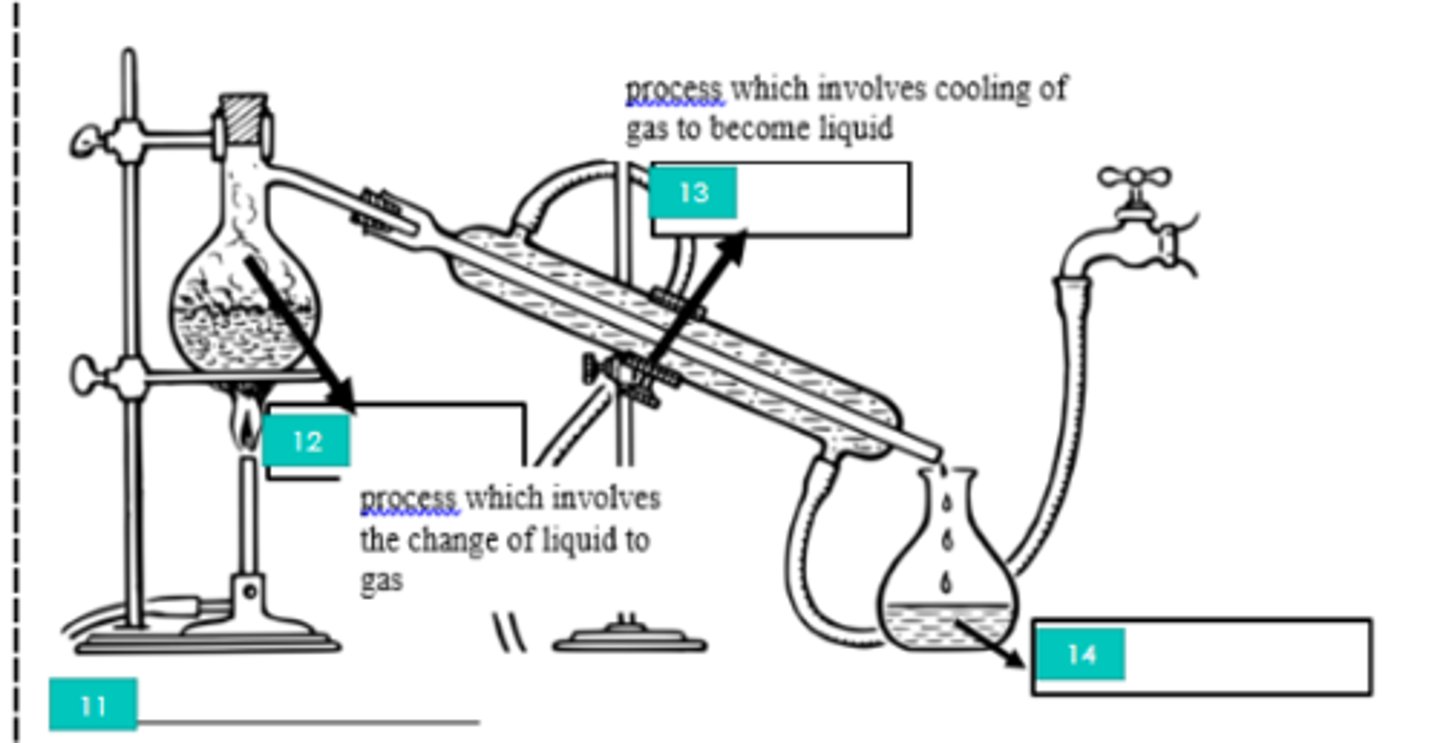
Distillate
14?
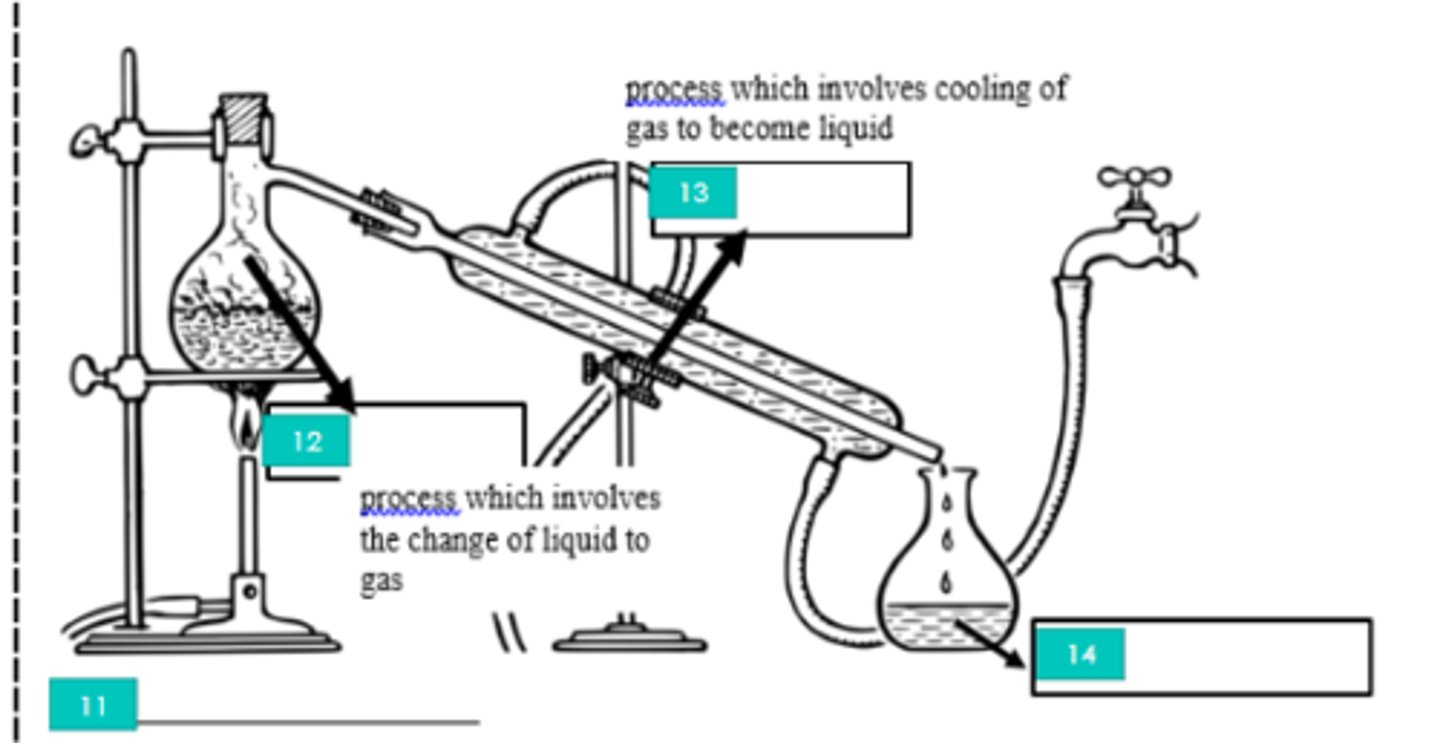
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Bronze
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Air
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
NaCl (aq)
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Oil and water
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Toothpaste
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Muddy water
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Brass
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Dental amalgam
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Alcohol and water
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Whipped cream
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Iron filings and sulfur
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Milk
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Blood
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Concrete
Heterogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Smoke
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Steel
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Brine
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Vinegar
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Jelly
Homogeneous mixture
Identify if it is a homogeneous or heterogeneous mixture:
Ink
Sieving
Separating method used to separate components of mixture with different sizes
Magnetism
Separating method used to separate materials with different magnetic property
Decantation
Separating method for mixtures of liquid and heavy insoluble solids
For sand-water mixtures, it can be separated by gently pouring the water out of the container after the sand has settled at the bottom of the container
Decantate
The liquid collected after decantation
Evaporation
Done by continuously heating the solution, leaving behind the solid component of the mixture
Sublimation
Conversion of a substance from the solid to the gaseous state
Filtration
The process in which solid particles in a liquid or gaseous fluid are removed by the use of a filter medium that permits the fluid to pass through but retains the solid particles
Filtrate
The liquid collected after filtration
Chromatography
The method used to separate components of different degrees of solubility using a moving and stationary fluid
Can be used to separate pigments of ink
Distillation
Separates miscible liquids of different boiling points such as alcohol and water
Distillate
The liquid collected after distillation
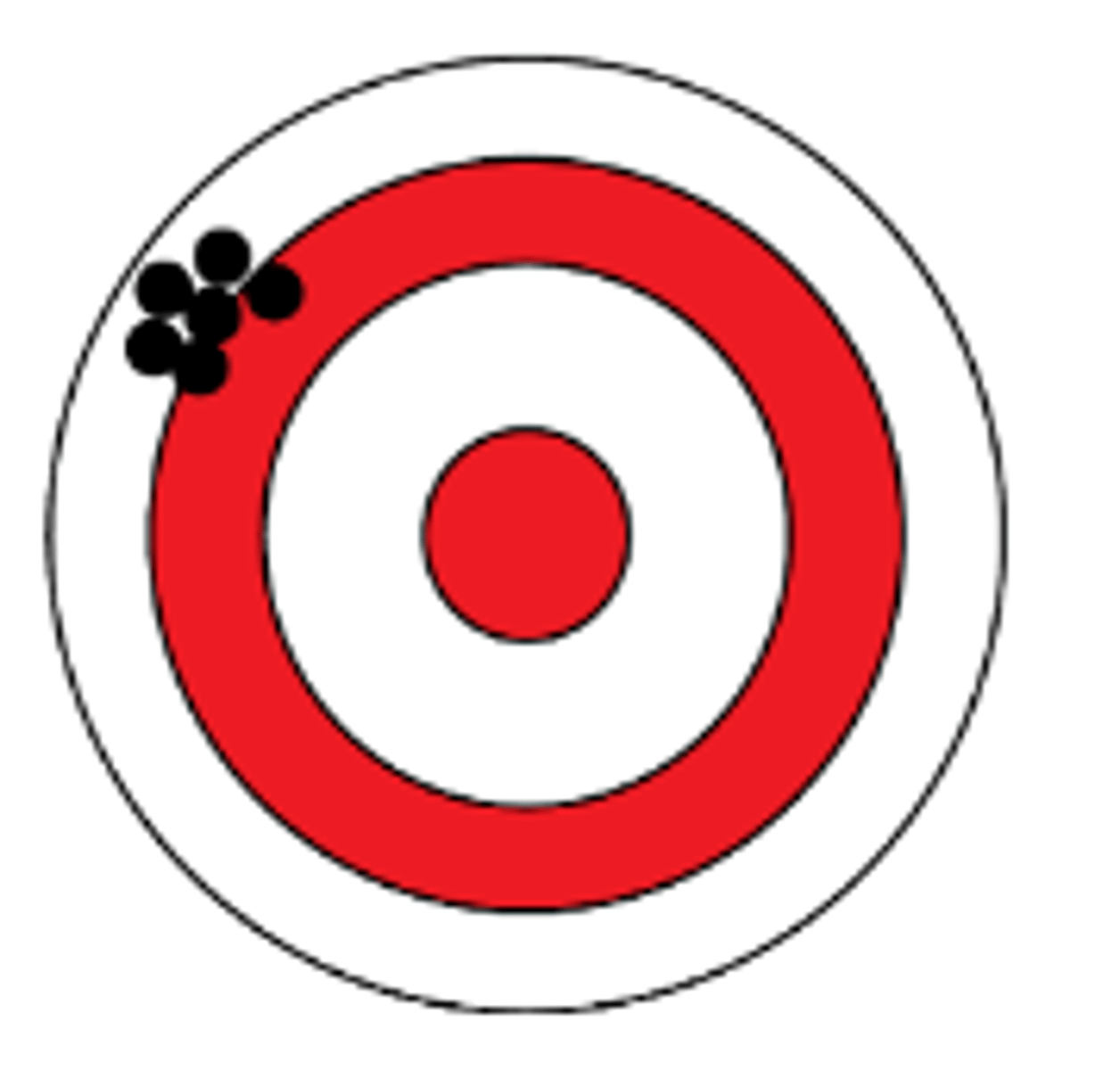
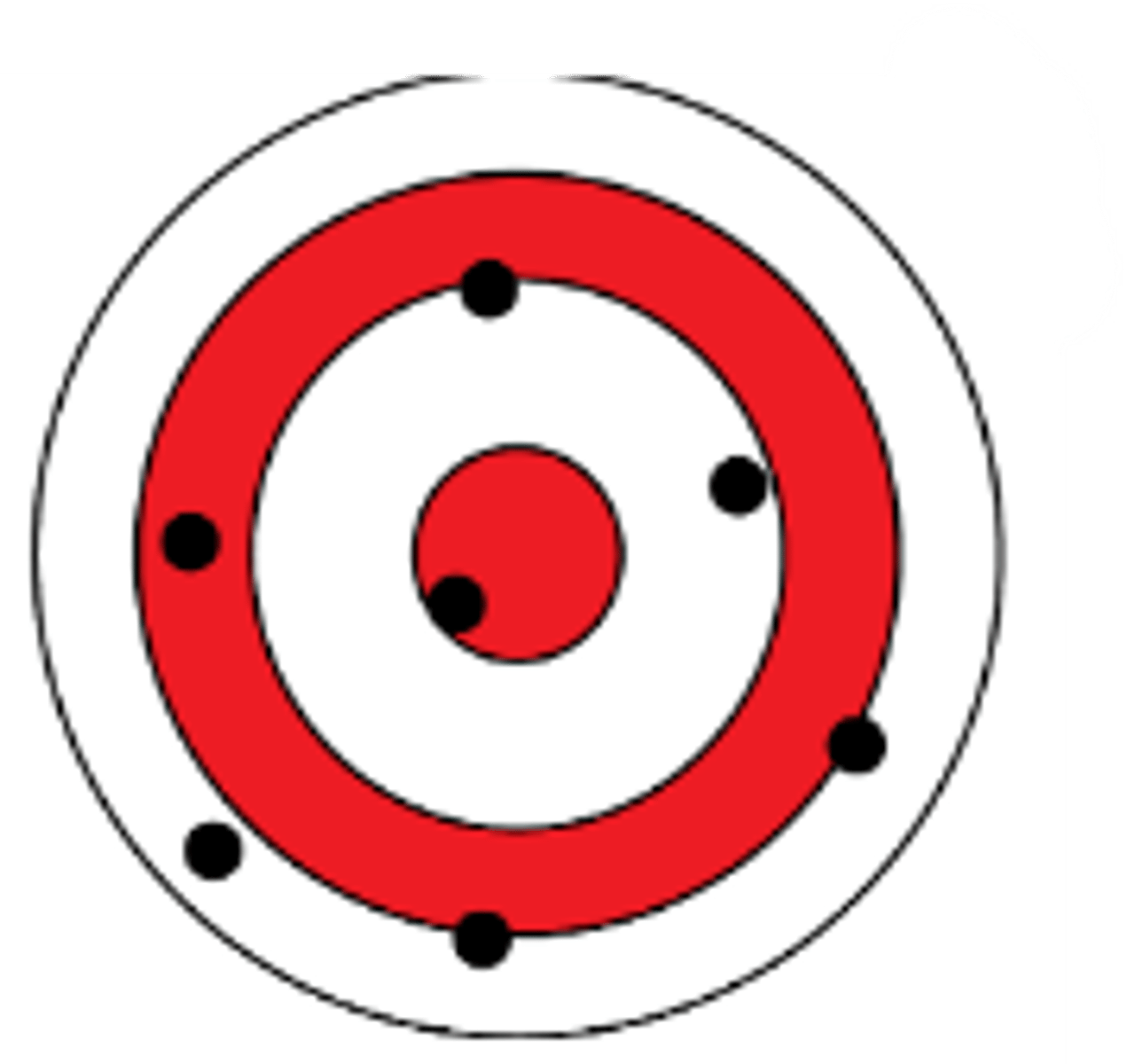
Accuracy
Indicates how close a measurement is to the true or accepted value
Precision
Refers to the closeness of measurements within a set of data
Getting similar results each time, even if it's not close to the actual value
High accuracy, high precision
Indicate the accuracy and precision
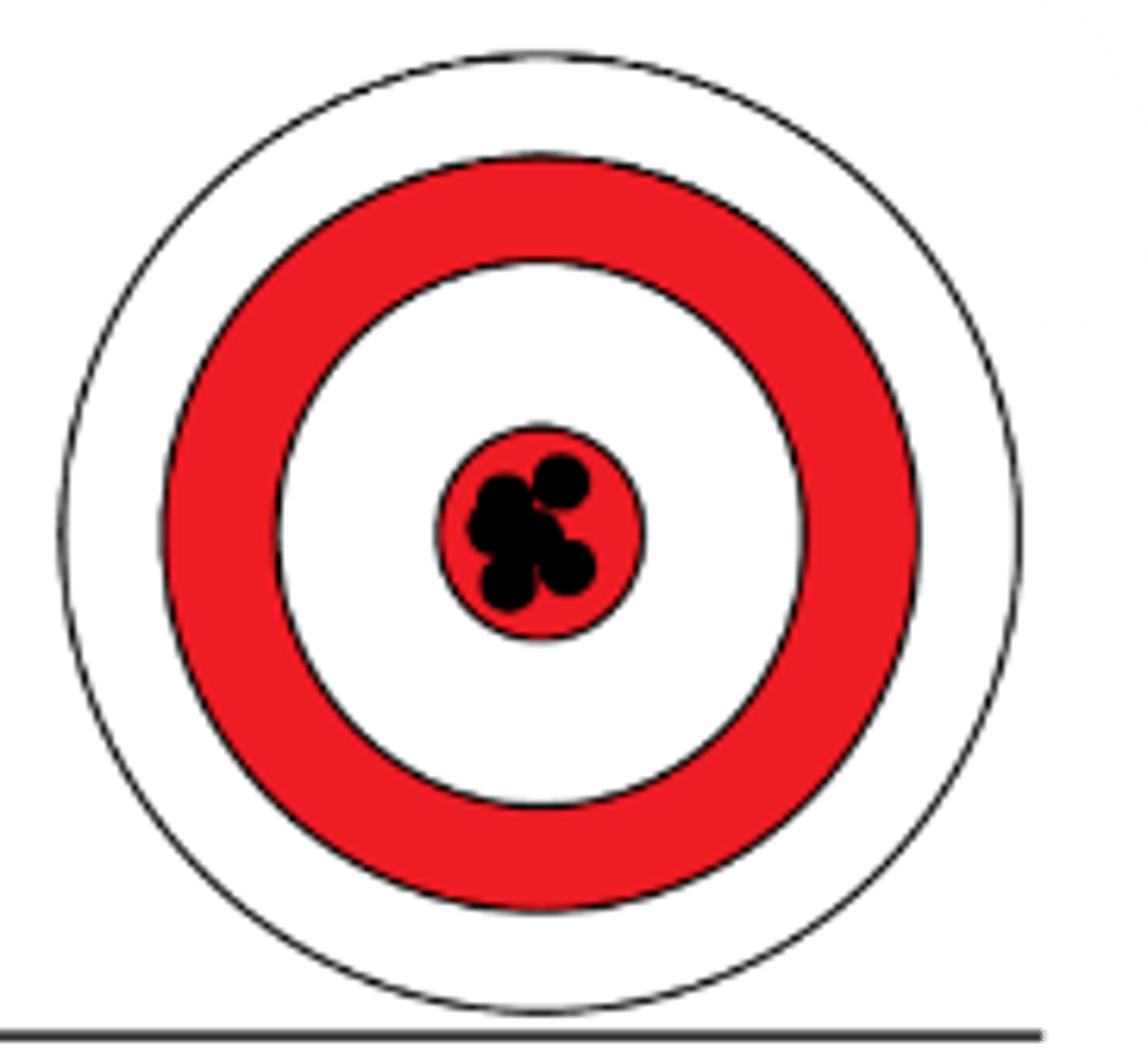
High accuracy, low precision
Indicate the accuracy and precision
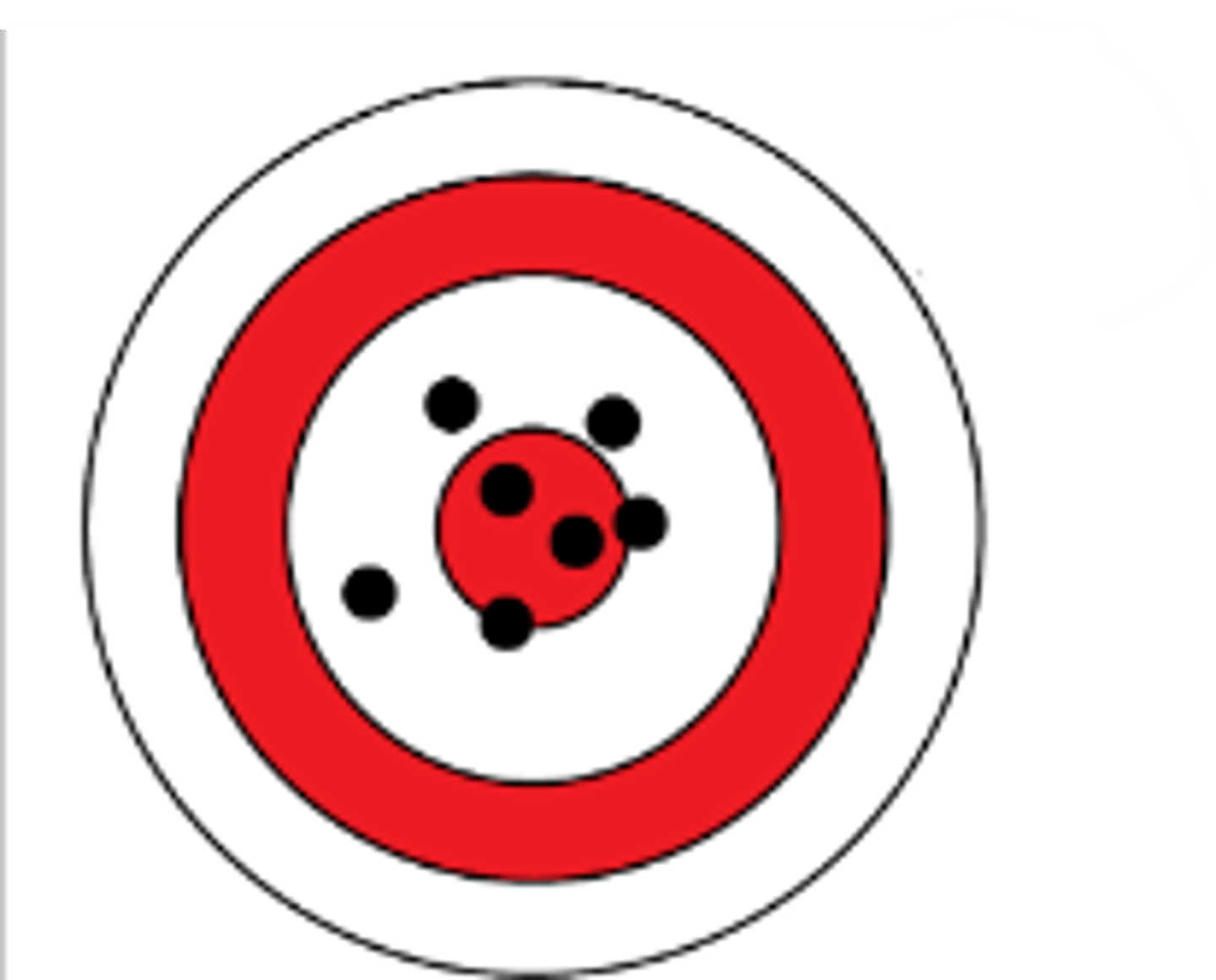
Low accuracy, high precision
Indicate the accuracy and precision
Low accuracy, low precision
Indicate the accuracy and precision
Significant figures
Indicate the precision of a number by showing which digits in a measurement are meaningful and reliable
Mass
Defined as the amount of matter in an object
Fixed quantity that is independent of the object's location
Weight
It is the pull of gravity on an object and depends on the object's location
Can be measured with: digital balance, triple beam balance, and analytical balance
Volume
The amount of space occupied by an object
Meniscus
The curvature in the interface of a liquid in any glass container
V = l x w x h
Formula for volume of regularly shaped solid?
Water displacement method
Formula for volume of irregularly shaped solid?
Density
An intrinsic property of matter, it is the mass (m) of an object per unit volume (V) it occupies = m/v
Analytical balance
A balance that is accurate to three decimal places
Top-loading/Platform balance
A balance that is accurate to two decimal places
Triple-beam balance
A balance that is accurate to one decimal places
W = Mg
Formula for weight?