2.4- Enzymes
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
What is an enzyme
a biological catalyst that speeds up rate of reaction by lowering activation energy
remain unchanged and can be re used
activity affects both structure and function within cells, tissues and organs
structure of enzymes
long chains of amino acids
folds into specific shape (tertiary structure) that determines shape of active site
globular protein
temporary bonds formed with substrate to form enzyme-substrate complex
anabolic reaction
bond making
two substrate molecules held closely together to reduce repulsion so they can bond easily
catabolic reaction
bond breaking
fitting in active site puts strain on bonds so molecule breaks up easily
intracellular enzymes
work within cells and organelles
example of intracellular enzyme
catalase breaks down hydrogen peroxide
2H2O2→ 2H2O + O2
what is a metabolic pathway
a sequence of enzyme catalysed reactions where the product of one reaction is the substrate of the next
extracellular enzymes
some enzymes are secreted from cells where they are made and act on substrates outside of the cells
example of extracellular enzyme
amylase secreted from salivary glands acting in the mouth and secreted from pancreas acting in small intestine
trypsin made in pancreas and acts in small intestine
what is a cofactor
a non-protein molecule that helps catalyse reactions
types of cofactor
organic
inorganic
what is a cosubstrate
ions that bind to substrate to form correct shape to bind to active site of enzyme
coenzymes
type of cofactor
organic non-protein molecules
often bind temporarily to active site just before/ as substrate binds
coenzymes changed during reaction→ need to be recycled into original state
prosthetic groups
type of cofactor
inorgnic non-protein molecule
permanently bound to an enzyme
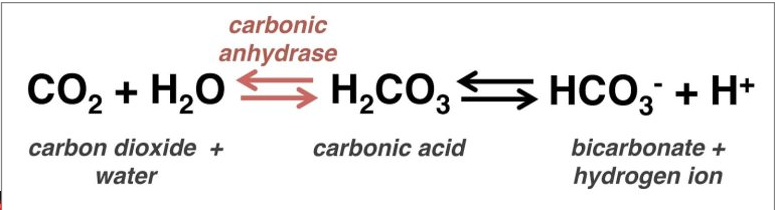
prosthetic group example
zinc ion permanently bound to carbonic anhydrase
other cofactors
inorganic non protein molecules e.g. mineral ions
not permanently bound to enzyme
temporarily associate with either the substrate or the enzyme during reaction to increase the rate of reaction
effect of cofactors on structure + e.g.
some cofactors change charge or distribution of charge on surface of either substrate or enzyme
makes the enzyme-substrate complex easier to form
e.g. amylase only functions if chloride ions are present
lock and key model of enzyme action
the substrate fits into the enzyme exactly in the same way that a key fits into a lock
the active site and substrate have a complementary shape
induced fit model of enzyme action
active site and enzyme are not completely complementary- active site is not a fixed shape
active site changes shape to form enzyme substrate complexes→ flexible
effect of enzymes on activation energy
puts strain on substrate bonds, weakening them
lowers activation energy required as less energy required to break bonds
factors affecting rate of enzyme controlled reaction
temperature
pH
substrate concentration
enzyme concentration
concentration of inhibitors
effect of temperature on enzyme controlled reactions up to optimum temp
higher temp=particles have higher Ek= more frequent collisions= more successful collisions= more enzyme substrate complexes formed
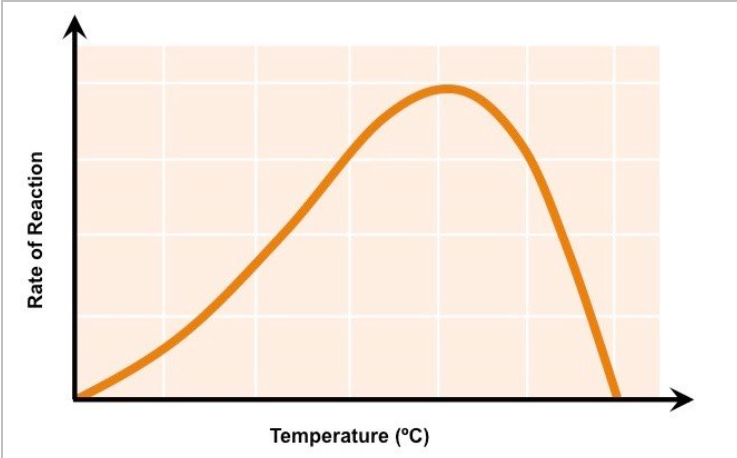
optimum temperature
temperature at which rate of reaction is at its maximum
effect of temp on enzymes past optimum
hydrogen bonds begin to break down
tertiary structure of protein changes
shape of active site changes→ no longer complementary to substrate
no ESC formed so reaction stops
temperature coefficient
the increase in rate of process when temp is increased by 10°C
Q10=rate of reaction at (T+10)°C/rate of reaction at T°C
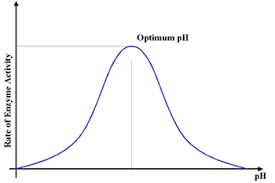
effect of pH on enzyme activity
each enzyme has an optimum pH
if lower than optimum pH, excess H+ ions disrupt H- bonds and ionic bonds→ impacts shape of active site
H+ ions will alter charges on amino acids in active site → interferes with binding of substrate to active site
same above above optimum pH with OH- ions.
calculating pH
pH=-log10 [H+]
what is a buffer
a substance that resists a change in pH
accept or donate protons to maintain pH
effect of substrate concentration on enzymes
lower substrate concentration:
too few substrates to occupy all available active site
enzymes have lowered chances of successful collisions
intermediate substrate concentration:
more active sites occupied at any one time
higher rate of reaction→ Vmax
higher substrate concentration:
no effect as all active sites full
enzyme concentration is a limiting factor
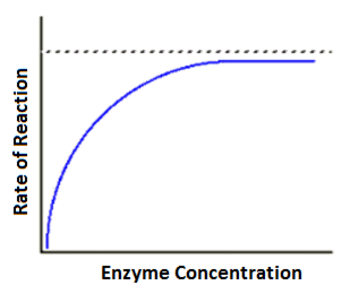
effect of enzyme concentration on enzymes
low enzyme concentration:
few enzyme molecules to allow all substrate molecules to find an active site
intermediate enzyme concentration:
all substrate molecules can occupy an active site at once
rate of reaction increased to Vmax
High enzyme concentration:
no effect→ all substrates being catalysed
substrate concentration is a limiting factor
types of inhibitors
competitive
non-competitive
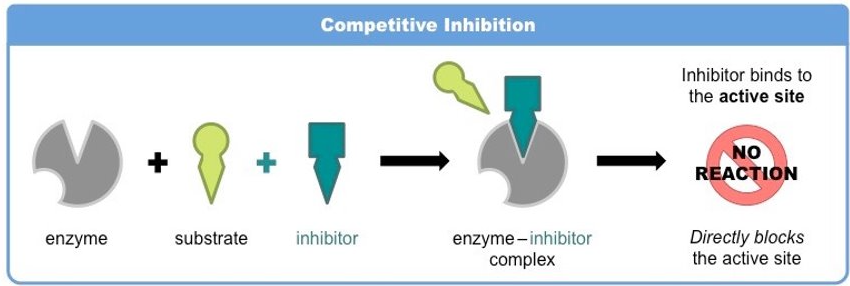
competitive inhibitors
usually not permanent→ reversible
in competition for active site→ if substrate concentration is increased, inhibitor effect is reduced
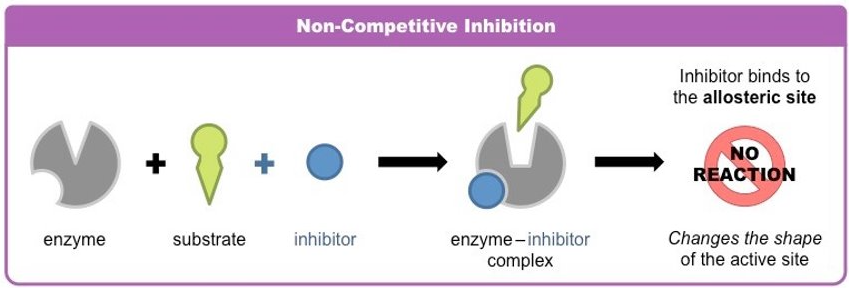
non-competitive inhibitors
usually not permanent→ reversible
binds to allosteric site→ changes shape of active site, preventing ESCs forming
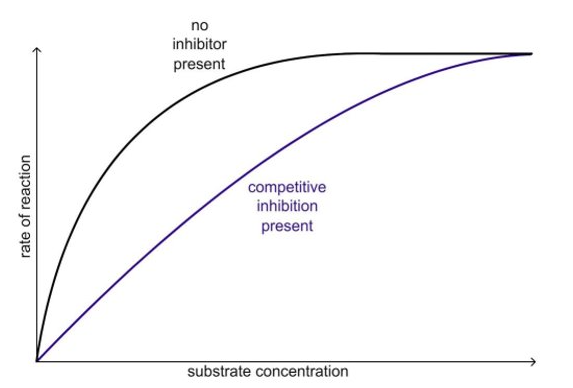
effect of competitive inhibitors
overcome by increasing substrate conc.
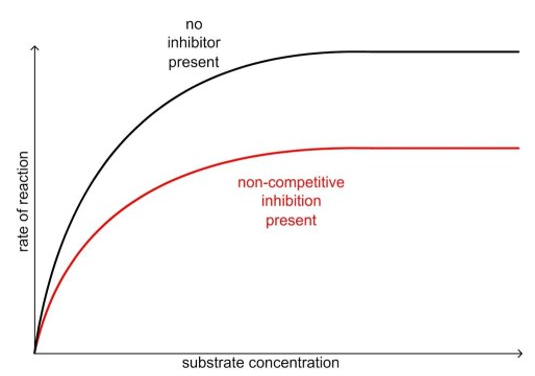
effect of non-competitive inhibitors
not affected by increasing substrate conc.
non-reversible inhibitors
bind permanently to enzyme
strong covalent bonds formed between enzyme and inhibitor
end product inhibition
allows control of metabolic pathways so there isn’t too much or too little substance in cells
end product acts as an inhibitor of an enzyme earlier in the pathway