CSF Exam 3
1/251
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
252 Terms
Signal Transduction
The process by which cells communicate with each other and respond to external stimuli through a series of molecular events, often involving receptors and second messengers.
Contact Dependent Signaling
A form of cell communication where cells must be in direct contact with each other to transmit signals, often through membrane-bound ligands and receptors.
Paracrine Signaling
A type of signaling in which cells secrete signals that affect nearby target cells, allowing localized communication.
Autocrine Signaling
A form of signaling where a cell secretes a chemical messenger that binds to receptors on its own surface, thereby affecting its own activities.
Neuronal Signaling
A specialized form of communication between nerve cells (neurons) where neurotransmitters are released at synapses to transmit signals across gaps to target cells.
Endocrine Signaling
A type of signaling where hormones are secreted into the bloodstream by endocrine glands and travel to distant target cells, facilitating long-range communication.
Nitric Oxide gas released by endothelial cells in the
blood vessels diffuses to induce relaxation of
adjacent smooth muscle cells. This is an example
of___________ signaling.
paracrine
Extracellular Molecule Binding Locations
Cell Surface Receptors, Intracellular Receptors
Effect of Different Combination of Same Signals
Growth, Division, Survival, Differentiation. Apoptosis Utilizes a unique combination of signals, not any signal common to those listed before.
Same Signal in different target cells
can produce varied responses in each cell type, depending on their specific receptor types and intracellular pathways.
During nervous-system development in Drosophila, the membrane-bound
protein Delta acts as an inhibitory signal to prevent neighboring cells from
developing into neuronal cells. Delta is involved in ______________ signaling.
Contact Dependent
Nerve Cell Production
Delta and Notch receptors bind, differentiating one endothelial cell in the center while inhibition all surrounding
If signaling molecules act on the same cells that release them, it is an example
of _____________ signaling.
autocrine
Ion Channel Coupled Receptors
A class of cell surface receptors that mediate rapid responses by forming ion channels in the plasma membrane. These receptors allow ions to flow across the membrane, initiating cellular signaling.
G Protein Coupled Receptors
A large class of cell surface receptors that activate intracellular signaling pathways through the activation of G proteins. These receptors play a key role in various physiological processes and are targets for many pharmaceuticals.
Enzyme Coupled Receptors
A class of cell surface receptors that, upon binding a ligand, activate intrinsic enzymatic activity or associate with enzymes to trigger cellular responses. These receptors are involved in various signaling pathways that regulate cellular functions.
G Protein Structure
The three main components of a G protein: the alpha, beta, and gamma subunits, which work together to transmit signals from activated receptors to downstream effectors.
GEF
Guanine nucleotide exchange factor, which facilitates the exchange of GDP for GTP on G proteins, thereby activating them in signaling pathways.
GAP
A GTPase-activating protein that accelerates the hydrolysis of GTP to GDP, thereby inactivating G proteins and terminating signaling.
Which amino acid residues can be phosphorylated?
There are three primary amino acid residues that can be phosphorylated: serine, threonine, and tyrosine. Phosphorylation of these residues plays a crucial role in regulating protein function and signaling pathways.
Kinase
Phosphorylates Target Protein
Phosphatase
An enzyme that removes phosphate groups from proteins, reversing the action of kinases and regulating protein function.
Monomeric GTPases are in “ON-State(active)” upon binding to
GTP
Scaffold Protein
A type of protein that provides a structural framework, facilitating the assembly of multiple proteins into signaling complexes, enhancing their interactions and specificity.
Interaction Domains
Specific regions on proteins that facilitate binding to other proteins or molecules, playing key roles in signaling pathways and cellular interactions.
SH2 Domain
A protein interaction domain that specifically binds to phosphorylated tyrosine residues on target proteins (phosphotyrosine).
PTB
A protein interaction domain that recognizes and binds to phosphorylated tyrosine residues (phosphotyrosine).
SH3
A protein interaction domain that binds to proline-rich sequences in target proteins, often involved in mediating protein-protein interactions.
PH
A protein interaction domain that recognizes phosphorylated serine or threonine residues, binding to charged head groups of phosphoinositides.
Variables Affecting Response to a signal
Response timing, sensitivity, dynamic range of signaling, persistence of response, signal processing, integration, coordination of multiple responses
Coincidence Detector
A mechanism or system in signaling pathways that requires two or more signals to elicit a response, ensuring specificity and preventing unwanted activation. (AND gate)
What causes a slow response to extracellular signaling?
When the signal causes gene expression changes, altering protein synthesis
What causes a fast response to extracellular signaling?
Altering protein function, such as phosphorylation
Which of these occur more rapidly in response to a signal?
A- Changes in protein phosphorylation
B- Changes in mRNA synthesis
Changes in protein phosphorylation
Positive Feedback
A process where the output of a system enhances or increases its own production, often leading to a greater response.
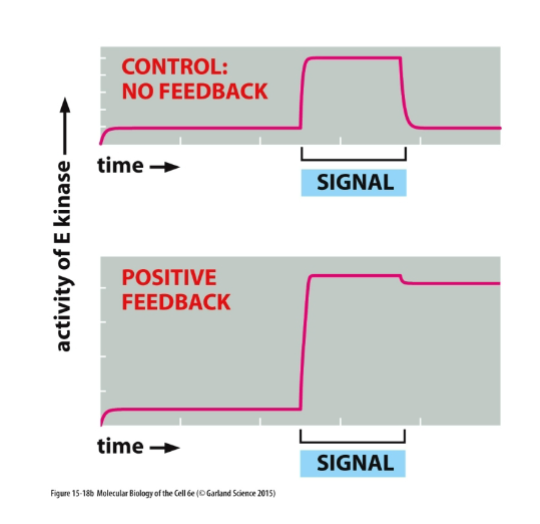
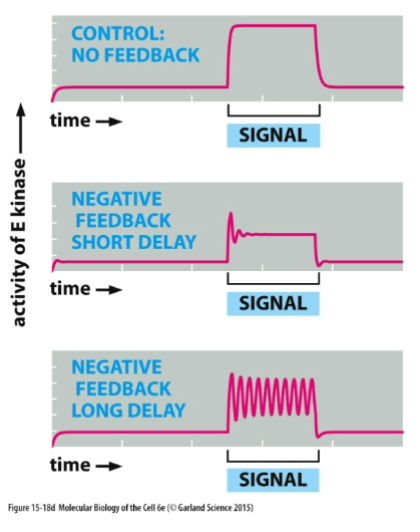
Negative Feeback
A regulatory mechanism in which a change in a variable triggers a response that counteracts the initial change, thereby maintaining homeostasis.
Do cells respond to signals gradually at the same time, or one at a time in an “all or nothing” fashion?
All or nothing
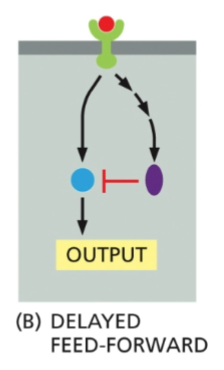
Delayed Feed Forward
A very far downstream effect of a receptor is inhibition the next enzyme in the pathway, causing a delayed inhibition
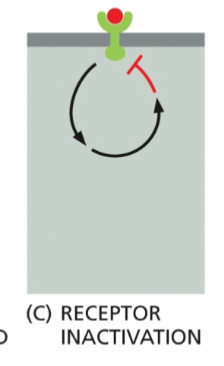
Receptor Inactivation
The receptor itself is inhibited by the downstream effects of its own signaling pathway
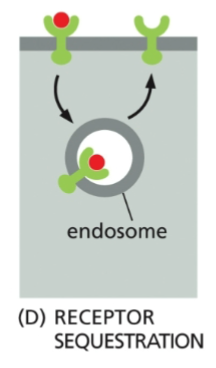
Receptor Sequestration
The receptor is eaten by an endosome, but then returned to the membrane
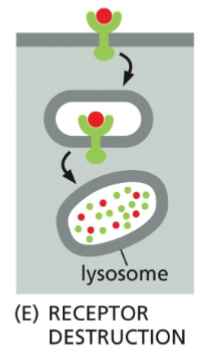
Receptor Destrucion
The receptor is permanently removed from the cell membrane, usually through lysosomal degradation.
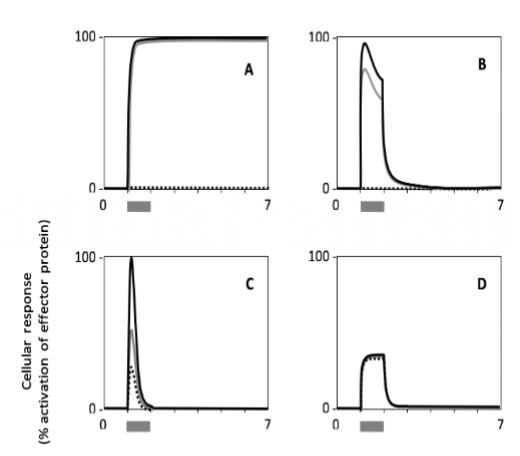
Which has the fastest signal adaptation?
C
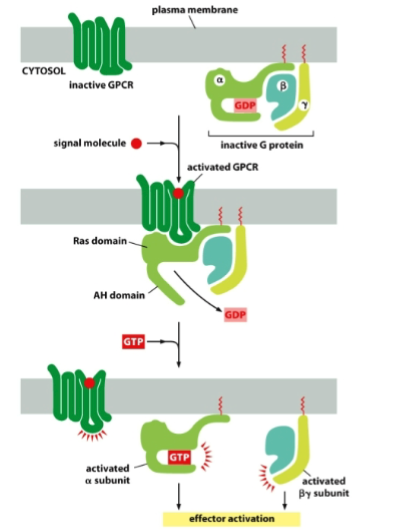
GPCR Structure
7 pass transmembrane as alpha helices, receptor binds to G protein on the cytoplasmic side, and is activated by ligand binding on the extracellular side.
Structure of an Inactive G protein
Alpha and Gamma are bound to the plasma membrane on the cystolic side, while beta is held in between them, the alpha subunit also has a GDP held in the AH domain
What happens when you activate GPCR?
The GPCR undergoes a conformational change, activating the associated G protein by exchanging GDP for GTP on the alpha subunit, separating the alpha subunit apart from the beta and gamma subunits and activating downstream effects
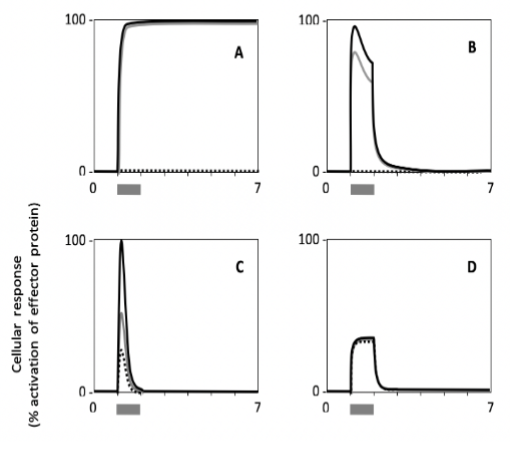
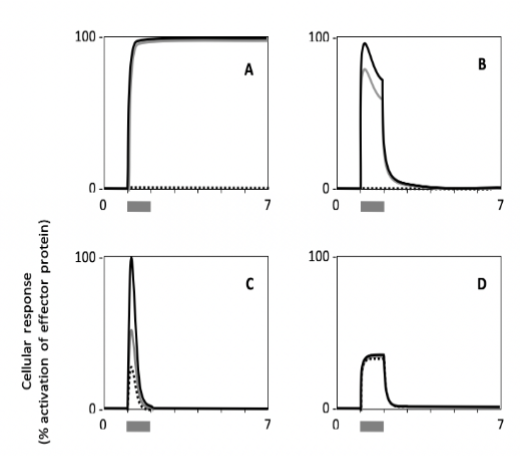
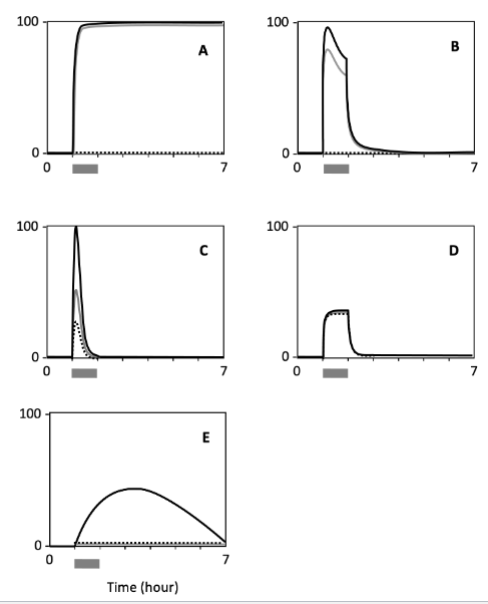
Which cell type shows a response with the highest persistence?
A
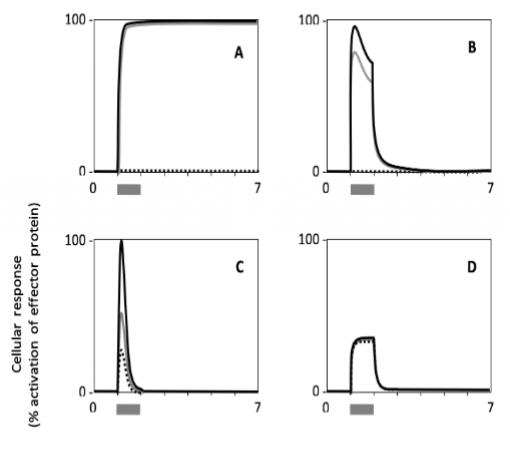
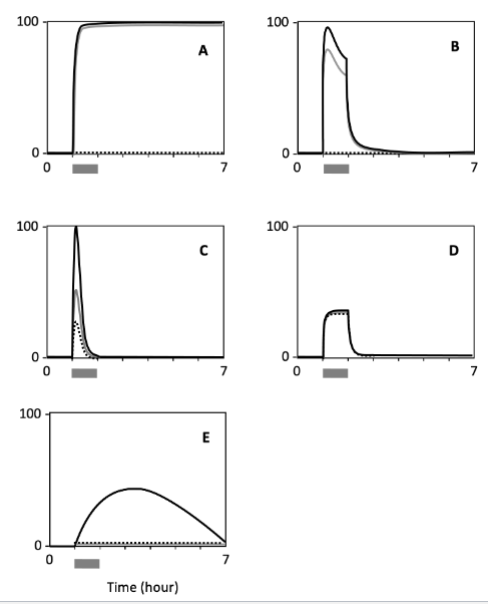
Which cell shows the lowest sensitivity to the signal?
E
Which best describes a GPCR?
A- All receptors of this class are polypeptides with seven transmembrane segments.
B- Alter the membrane potential directly by changing the permeability of the plasma membrane.
C- Must be coupled with intracellular monomeric GTP-binding proteins
A - All receptors of this class are polypeptides with seven transmembrane segments.
The length of time a G protein will signal is determined by _______.
A- the activity of phosphatases that dephosphorylate Gα.
B- the activity of protein phosphatases that turn GTP into GDP.
C- the GTPase activity of Gα.
C- the GTPase activity of Gα.
Arrestin
A protein that regulates GPCR activity by binding to phosphorylated receptors, preventing further signaling.
Trans Autophosphorylation
A process where receptor tyrosine kinases phosphorylate themselves on tyrosine residues, activating their kinase activity.
Likely effect of a RAS mutation preventing hydrolysis
of GTP to GDP, leading to persistent activation of downstream signaling pathways, extremely likely cause of cancerby continuously activating RAS signaling, which promotes uncontrolled cell growth and division.
PI 3 Kinase
A lipid kinase that phosphorylates phosphatidylinositol, leading to the activation of the Akt signaling pathway, which promotes cell survival and growth.
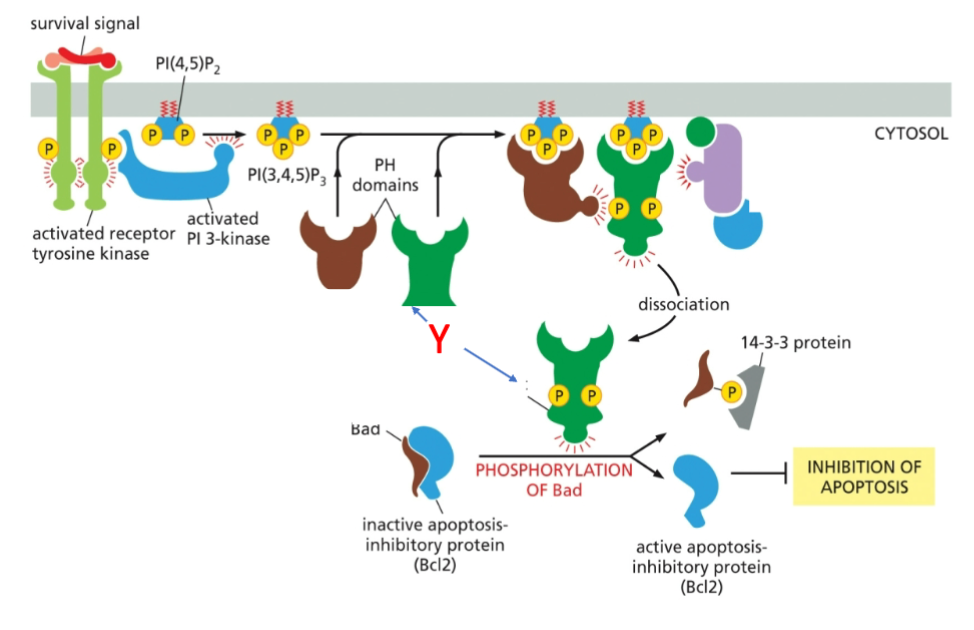
How does an active Akt promote cell survival
An active Akt promotes cell survival by inhibiting pro-apoptotic factors (BAD) and stimulating the expression of anti-apoptotic proteins, allowing cells to evade programmed cell death.
Which of the following mechanisms is not directly involved in inactivating an
activated RTK (Receptor Tyrosine Kinase)?
A- dephosphorylation by serine/threonine phosphatases
B- dephosphorylation by protein tyrosine phosphatases
C- removal of the RTK from the plasma membrane by endocytosis
D- digestion of the RTK in lysosomes
A- dephosphorylation by serine/threonine phosphatases
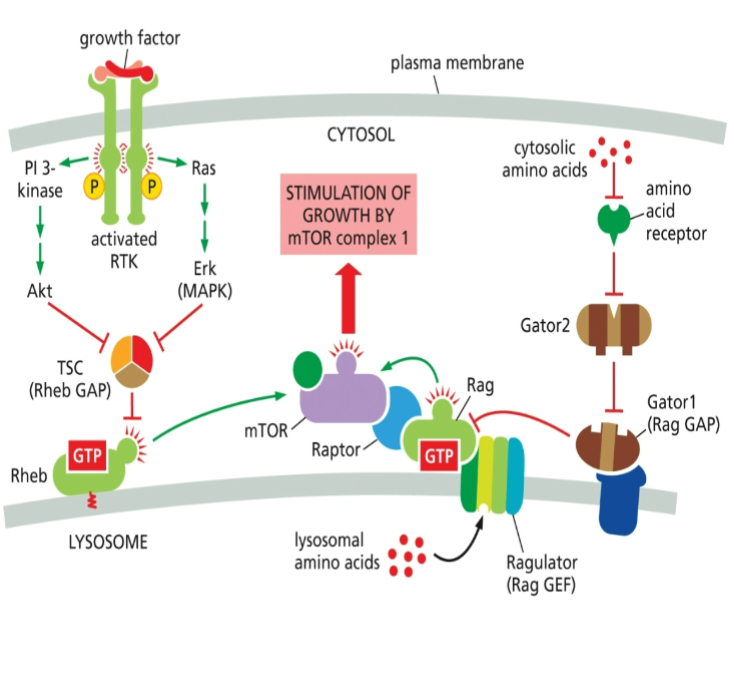
Effect of amino acids on PI 3 Kinase Akt mTOR pathway
Amino acids activate the PI 3 Kinase Akt mTOR pathway, enhancing protein synthesis and cell growth, primarily by promoting mTORC1 signaling.
Effect of growth factor on PI 3 Kinase Rheb mTOR signaling
Growth factors activate PI 3 Kinase, which enhances Rheb activity, leading to mTOR signaling activation, promoting cell growth and metabolism.
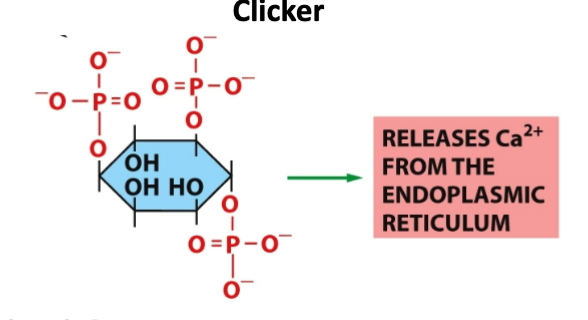
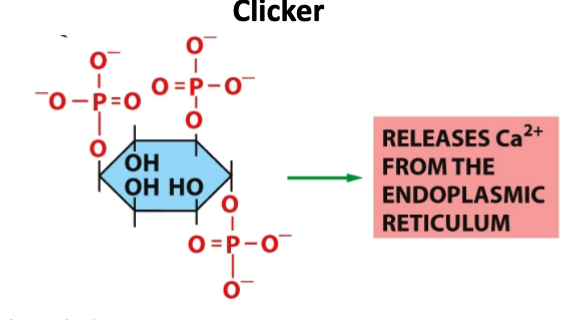
What is this molecule?
A- Phosphatidylinsoitol-3-phosphate
B- Inositol 1,4,5 triphosphate
C- Phosphatidylinsoitol- 4,5-bisphosphate
D- Phosphatidylinsoitol- 3, 4,5-triphosphate
B- Inositol 1,4,5 triphosphate
Steps of Notch processing and activation
Cleavage at Site 1 in Golgi
Transport to plasma membrane
binding to delta, endocytosis of delta notch fragment complex and cleavage at site 2
cleavage at site 3
notch tail migrates to nucleus
protein complex containing notch tail activates gene transcription
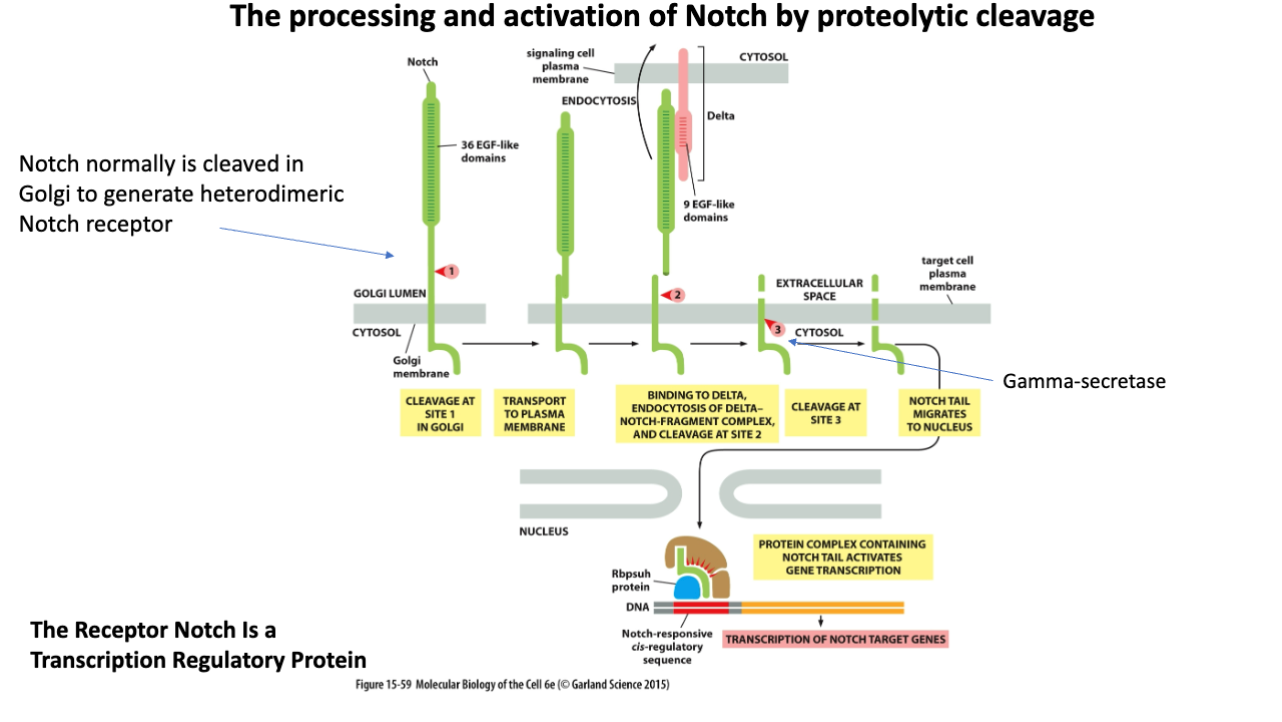
Is Notch Activation irreversible?
Notch activation is generally considered irreversible, as once the Notch intracellular domain translocates to the nucleus, it initiates gene transcription that modifies cell fate.
Locations for nuclear receptors
Nuclear receptors are typically found in the cytoplasm and nucleus of target cells, where they regulate gene expression in response to specific ligands.
How are nuclear receptors inactivated?
Receptors are usually inactivated by binding to inhibitory proteins complexes
How are nucelar receptors normally activated?
Binding of ligand induces a conformational change in the receptor that releases the inhibitory proteins and also causes the receptor to bind to co-activator proteins
There is no fundamental chemical distinction between signaling molecules that
bind to cell-surface receptors and those that bind to intracellular receptors.
A. True
B. False
False
What is the term for the protein that organizes groups of interacting intracellular signaling proteins into signaling complexes?
A. Intracellular receptor
B. Kinase cascade
C. Scaffold protein
D. Interaction domain
Scaffold Protein
What is the term for the compact protein modules found in many intracellular signaling molecules that bind to a particular structural motif in another protein (or lipid) molecule to which the signaling protein binds?
A. Cell-surface receptors
B. Ligands
C. Interaction domains
D. Scaffold proteins
Interaction domains
Negative feedback counteracts the effect of a stimulus and thereby abbreviates and limits the level of the response, making the system less sensitive to perturbations.
A. True
B. False
True
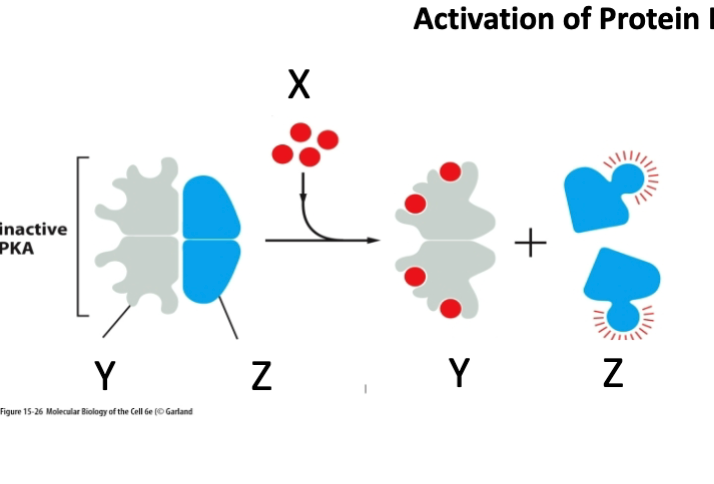
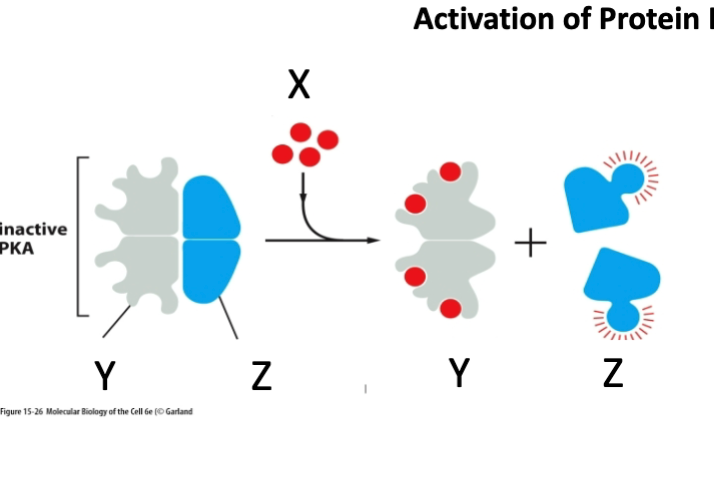
What is marked by letter X?
A- Regulatory subunit
B- cAMP
C- Catalytic subunit
cAMP
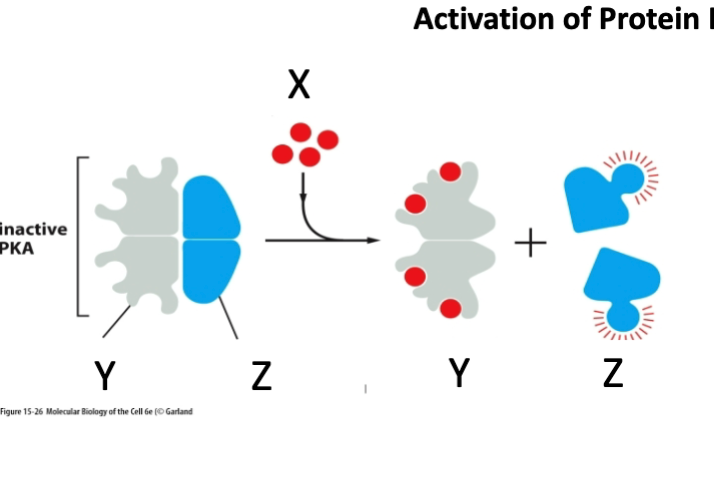
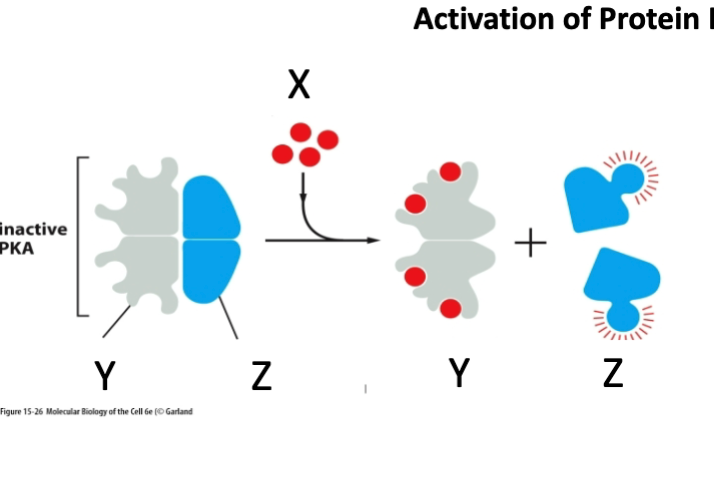
What is marked by letter Y
Regulatory subunit
Alterations in signaling in the pituitary gland can lead to human disease. The GH-releasing hormone (GHRH) stimulates release of growth hormone (GH) from the pituitary gland by binding to GHRH receptors, which are G-protein-coupled receptors. Excessive activity of the GHRH signaling leads to excessive release of growth hormone, which can lead to a form of gigantism.
Consider steps that could be taken to reduce GH release. Select if the following interventions indicated in blue below belong to A or B category.
•Block hydrolysis of GTP by G-alpha.
A. Decreases GH release
B. Increases GH release
Increases GH release
Alterations in signaling in the pituitary gland can lead to human disease. The GH-releasing hormone (GHRH) stimulates release of growth hormone (GH) from the pituitary gland by binding to GHRH receptors, which are G-protein-coupled receptors. Excessive activity of the GHRH signaling leads to excessive release of growth hormone, which can lead to a form of gigantism.
Consider steps that could be taken to reduce GH release. Select if the following interventions indicated in blue below belong to A or B category.
•Inhibit interaction of G-alpha with receptor
A. Decreases GH release
B. Increases GH release
A. Decreases GH release
Alterations in signaling in the pituitary gland can lead to human disease. The GH-releasing hormone (GHRH) stimulates release of growth hormone (GH) from the pituitary gland by binding to GHRH receptors, which are G-protein-coupled receptors. Excessive activity of the GHRH signaling leads to excessive release of growth hormone, which can lead to a form of gigantism.
Consider steps that could be taken to reduce GH release. Select if the following interventions indicated in blue below belong to A or B category.
•Activate phosphorylation of the receptor by GRK kinase
A. Decreases GH release
B. Increases GH release
Decreases GH release
Alterations in signaling in the pituitary gland can lead to human disease. The GH-releasing hormone (GHRH) stimulates release of growth hormone (GH) from the pituitary gland by binding to GHRH receptors, which are G-protein-coupled receptors. Excessive activity of the GHRH signaling leads to excessive release of growth hormone, which can lead to a form of gigantism.
Consider steps that could be taken to reduce GH release. Select if the following interventions indicated in blue below belong to A or B category.
•Block binding of arrestin to the receptor
A. Decreases GH release
B. Increases GH release
Increases GH release
Which of the following would increase phosphorylation of CREB by PKA?
A. blocking binding of the regulatory subunits to the catalytic subunits of PKA
B. blocking nuclear entry of PKA.
C. blocking ATP binding to the active site of PKA
D. blocking cAMP binding to the regulatory subunits of PKA
A. blocking binding of the regulatory subunits to the catalytic subunits of PKA
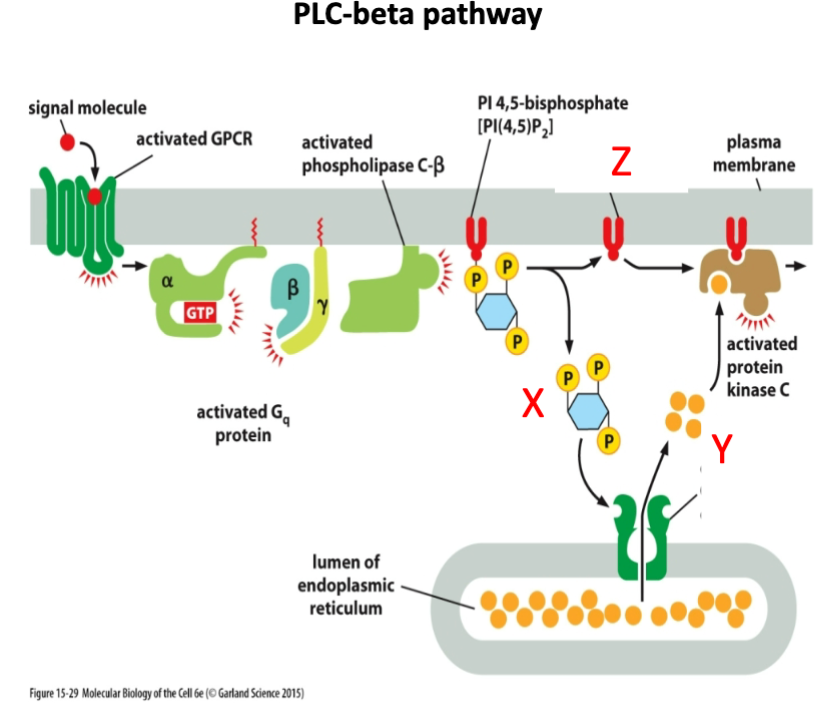
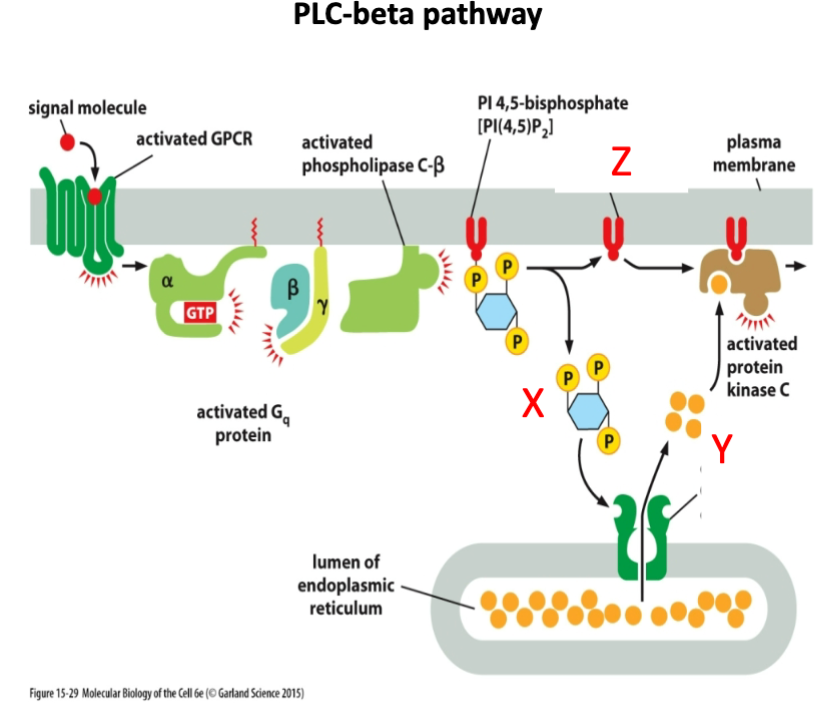
What is marked by letter X?
A- Inositol 1,4,5 triphosphate (IP3)
B- Diacyl glycerol
C- cAMP
Inositol 1,4,5 triphosphate (IP3)
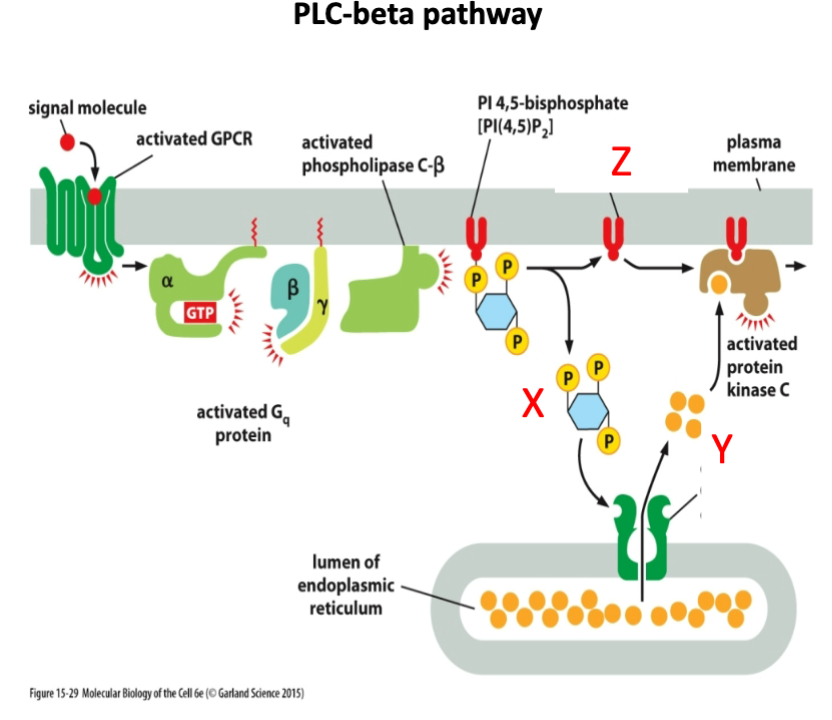
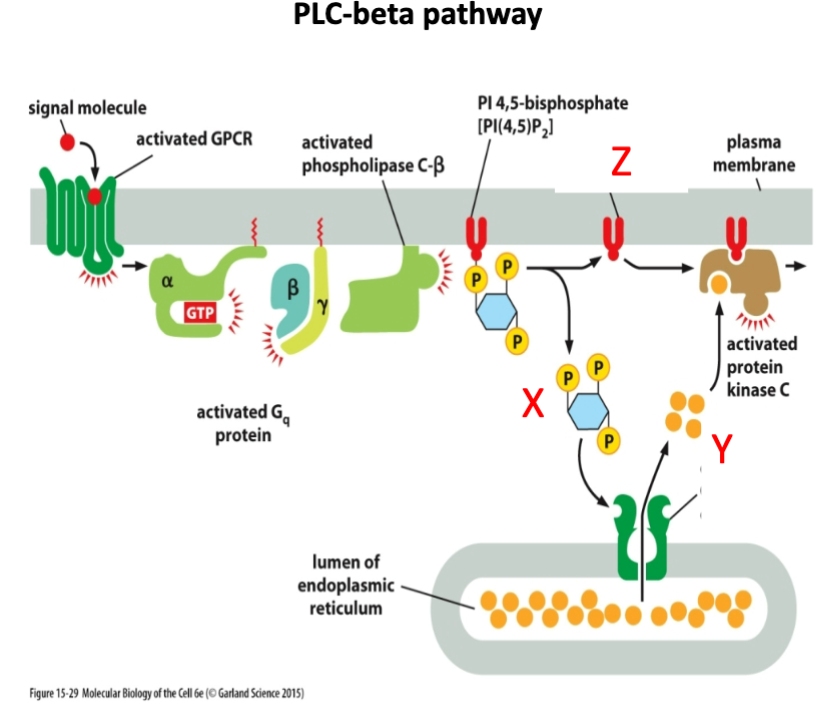
What is marked by letter Y?
A- cAMP
B- Calcium
C- cGMP
Calcium
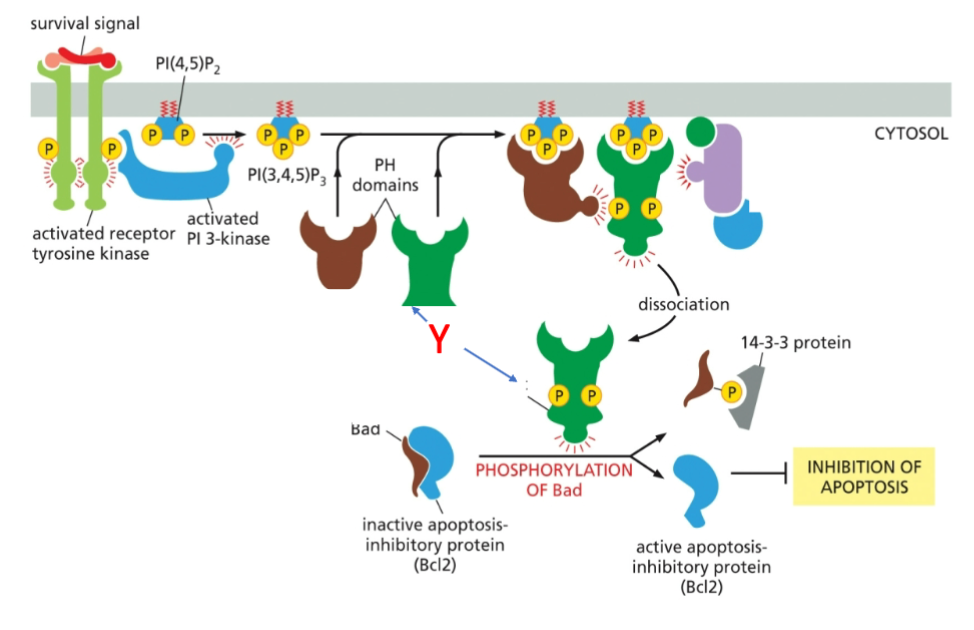
What is marked by letter Y?
A- AKT
B- PDK1
C- mTORC2
A- AKT
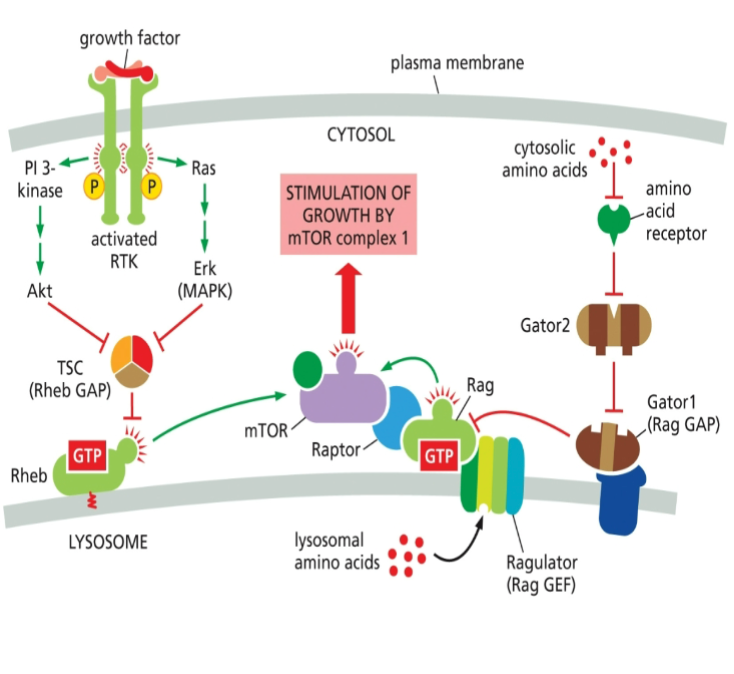
The mechanism of how Gator 2 inhibits Gator 1 has remained unknown. However, in 2023, researchers found that the E3 ligase subunit of Gator2 ubiquitinates a subunit of Gator1 and inhibits its GAP activity. What is the expected effect of this inhibition?
A. Rag remains bound to GDP
B. Ragulator activity is inhibited
C. mTORC1 kinase is activated
C. mTORC1 kinase is activated
Imagine that two protein kinases, PK1 and PK2, act sequentially in a kinase cascade that activates a set of target proteins bringing about a growth response. When either kinase is inactivated, cells do not respond to the extracellular signal. By contrast, cells containing a mutant form of PK1 that is permanently active respond even in the absence of an extracellular signal. Cells that contain both an inactivated PK2 and a permanently active PK1 respond in the absence of a signal.
Given these data, what is the order of action of PK1 and PK2?
A. PK1 activates PK2.
B. PK2 activates PK1.
B. PK2 activates PK1.
Imagine that two protein kinases, PK1 and PK2, act sequentially in a kinase cascade that activates a set of target proteins bringing about a growth response. When either kinase is inactivated, cells do not respond to the extracellular signal. By contrast, cells containing a mutant form of PK1 that is permanently active respond even in the absence of an extracellular signal. Cells that contain both an inactivated PK2 and a permanently active PK1 respond in the absence of a signal.
What outcome would you predict for a doubly mutant cell line with an activating mutation in PK2 and an inactivating mutation in PK1?
A. The doubly mutant cells would grow in the absence of signal.
B. The doubly mutant cells would not grow even in the presence of signal.
B. The doubly mutant cells would not grow even in the presence of signal.
Order of interphase?
G1, S, G2, M
Order of the M Phase?
Prophase, Prometaphase, metaphase, anaphase, telophase
What is DNA packed into?
Chromatin and chromosomes
What is G0?
A dormant stage where cells exit the cell cycle and do not actively prepare to divide. Cells in G0 can remain metabolically active but do not replicate their DNA. They can remain in this phase for years even.
When do cells enter the committed step? (Step or Restriction point)
G0 or G1
What is terminal differentiation?
The process where a cell undergoes specialization into a specific type, losing the ability to proliferate.
What is flow cytometry?
Flow cytometry is a laboratory technique that analyzes physical and chemical properties of cells in a fluid, often using fluorescently labeled antibodies
How do you label cells in S phase?
Nucleotides are incorporated in DNA during replication of S phase, special nucleotides that are modified to be visible can be then detected after they are incorporated, indicating a cell is in S phase.
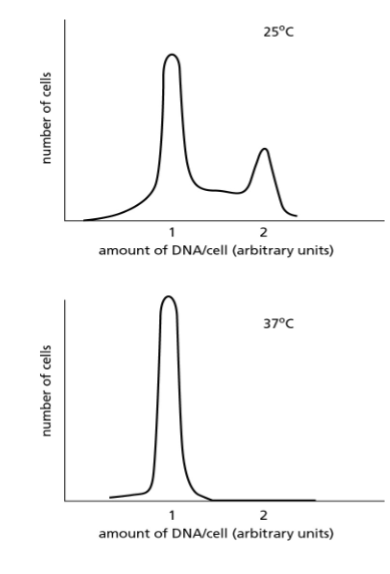
A mutant yeast strain stops proliferating when shifted from 25°C to 37°C. When these cells are analyzed at the two different temperatures, using a machine that sorts cells according to the amount of DNA they contain, the graphs in Figure below are obtained.
Which of the following would not explain the results with the mutant?
(a) inability to initiate DNA replication
(b) inability to begin M phase
(c) inability to activate proteins needed to enter S phase
(d) inappropriate production of a signal that causes the cells to remain in G1
b) inability to begin M phase
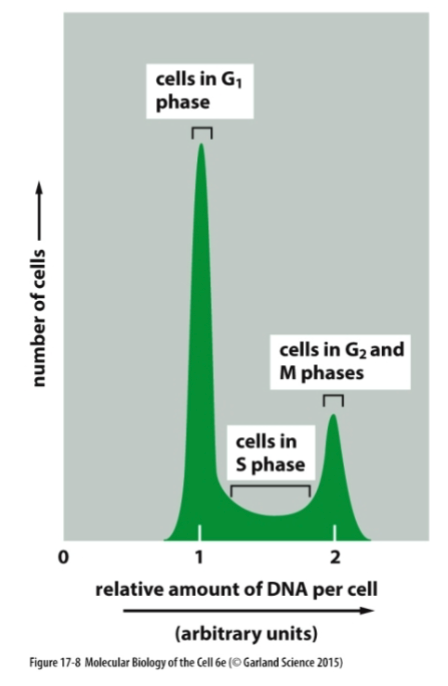
When do cells have the least, middle, and most DNA?
Cells have the least DNA in G1 phase, accumulate DNA during S phase, and have the most DNA during G2 and M phases as they replicate their DNA.
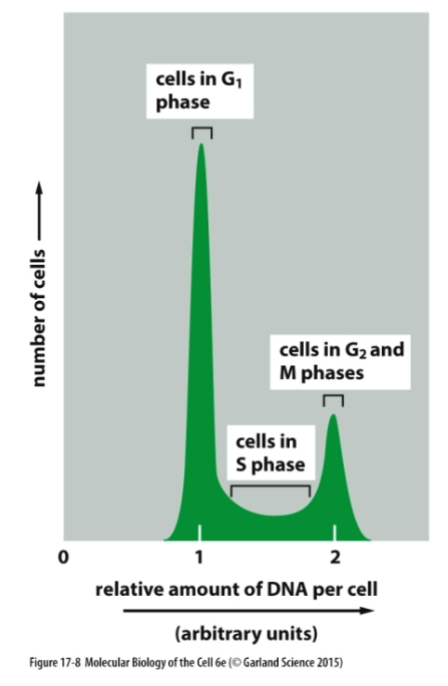
Which of the following events does not usually occur during interphase?
A- Cells grow in size.
B- The nuclear envelope breaks down.
C- DNA is replicated.
B- The nuclear envelope breaks down.
How is Cyclin (partially) activated?
By binding to CdK
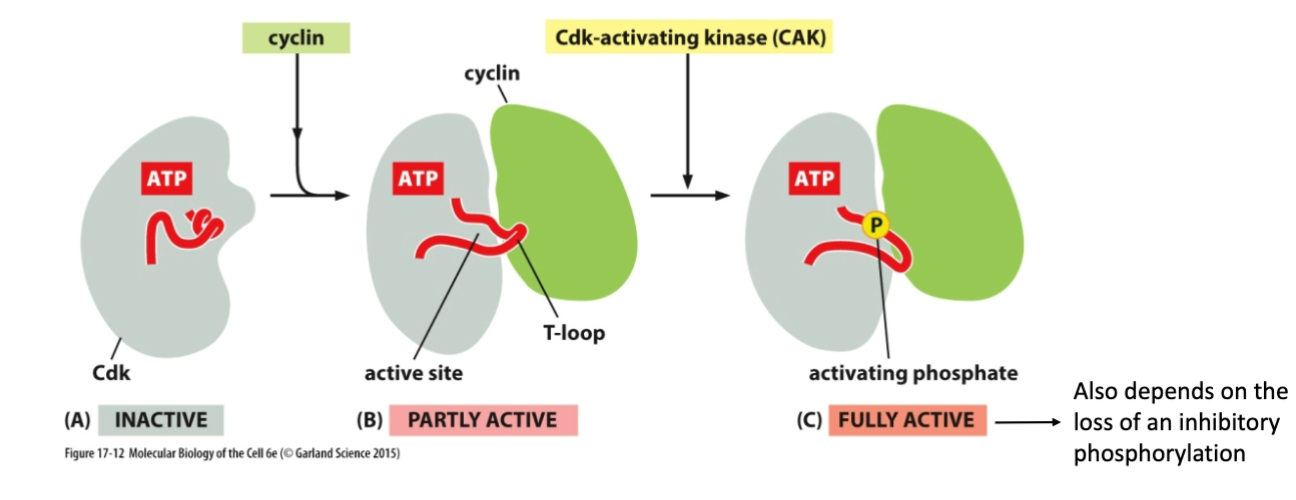
How is Cdk fully activated?
Binding to Cdk, getting phosphorylated at some sites, and getting dephosphorylated at other sites
What does the Cdk-cyclin complex do?
phosphorylates and regulates its substrates
Function of G1/S Cdk?
Regulates the transition from G1 phase to S phase in the cell cycle, promoting DNA replication.
Function of S-cdk?
helps launch S phase
What is the function of M-cdk?
promotes the transition from G2 phase to M phase, facilitating mitosis.
What does Wee1 Kinase do?
Phosphorylates Cdc25 by phosphorylating it
What do p27 and p21 do?
Inhibit cdk
Progression through the cell cycle requires a cyclin to bind to a Cdk because _________.
A- the cyclins are the molecules with the enzymatic activity in the complex.
B- the binding of a cyclin to Cdk is required for Cdk enzymatic activity.
C- cyclin binding inhibits Cdk activity until the appropriate time in the cell cycle
B- the binding of a cyclin to Cdk is required for Cdk enzymatic activity.