Chemistry - Chapter 2 (Nature of Matter)
0.0(0)
0.0(0)
Card Sorting
1/82
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
1
New cards
Matter
anything that has mass, occupies space, and has inertia
2
New cards
Extensive properties
qualities that are or depend upon the amount of the material. these do not help identify a material.
3
New cards
Intensive properties
qualities that
1. do not depend on the amount of material
2. help identify a material
it must have BOTH criteria to be an intensive property
1. do not depend on the amount of material
2. help identify a material
it must have BOTH criteria to be an intensive property
4
New cards
Physical properties
qualities that can be observed only where physical changes are allowed
5
New cards
Chemical properties
qualities that describe chemical changes or interactions between different forms of matter. these require a chemical change to observe or measure.
6
New cards
Chemical change
any change in which experimental evidence indicates that original substances (reactants) have been consumed and new substances (products) have been produced. a chemical change involves a re-organization of atoms, or molecules, by the breaking and reforming of chemical bonds.
7
New cards
Experimental evidence
8
New cards
Particle relationships
New properties emerge at and describe each level of organization
9
New cards
Kinetic energy
a measure of the energy of particles; any energy that cannot be stored; motion
10
New cards
Potential energy
stored energy
11
New cards
Thermal energy
kinetic energy of a material's particles
12
New cards
Temperature
measure of the AVERAGE kinetic energy in a sample of a material (this is because of random particle collisions)
13
New cards
States of matter
1. solid
2. liquid
3. gas
4. plasma (charged gas)
2. liquid
3. gas
4. plasma (charged gas)
14
New cards
Solid
- fixed shape
- fixed volume
- particles can only vibrate in place
- can't change places
- least KE and PE of the states of matter
- has the strongest attractive forces (stuck together strongly)
- fixed volume
- particles can only vibrate in place
- can't change places
- least KE and PE of the states of matter
- has the strongest attractive forces (stuck together strongly)
15
New cards
Liquid
- shape adopts its container
- particles touching each other (no space between particles)
- particles can move
- increase in PE and KE since the solid state
- particles touching each other (no space between particles)
- particles can move
- increase in PE and KE since the solid state
16
New cards
Gas
- huge increase in PE and KE
- space between particles (this is why gases can be compressed)
- shape and volume adapts to its container
- space between particles (this is why gases can be compressed)
- shape and volume adapts to its container
17
New cards
Kinetic Molecular Theory
KMT explains what happens to matter when the KE of particles changes.
1. all matter is made up of tiny particles
2. there is empty space between these particles
3. these particles are always in motion, but their freedom to move depends on their state of matter
4. these particles move because of energy. the amount of energy they have determines how fast and far they move.
1. all matter is made up of tiny particles
2. there is empty space between these particles
3. these particles are always in motion, but their freedom to move depends on their state of matter
4. these particles move because of energy. the amount of energy they have determines how fast and far they move.
18
New cards
Melting point
the point at which a solid changes into a liquid (intensive property)
19
New cards
Freezing point
the point at which a liquid changes into a solid (intensive property)
20
New cards
Boiling point
the point when vapor pressure is the same as the atmospheric pressure outside of the boiling liquid (intensive property)
21
New cards
Vapor pressure
the pressure of vapor inside a bubble during boiling (intensive property)
22
New cards
Volatility
how readily a liquid turns into a gas (intensive property)
23
New cards
Heat of Fusion
- endothermic event
- melting
- potential energy
- attractive forces overcome
- indicated in energy per amount (J/mol)
- intensive physical property
- melting
- potential energy
- attractive forces overcome
- indicated in energy per amount (J/mol)
- intensive physical property
24
New cards
Heat of Vaporization
- much more than heat of fusion
- massive increase in PE
- intensive physical property
- massive increase in PE
- intensive physical property
25
New cards
Heating curve
- if temp is going up, KE increase (no attractive forces overcome yet)
- when changing states, PE increases (attractive forces overcome)
particles gain KE and vibrate. when they have enough energy, they change states and overcome attractive forces
- when changing states, PE increases (attractive forces overcome)
particles gain KE and vibrate. when they have enough energy, they change states and overcome attractive forces
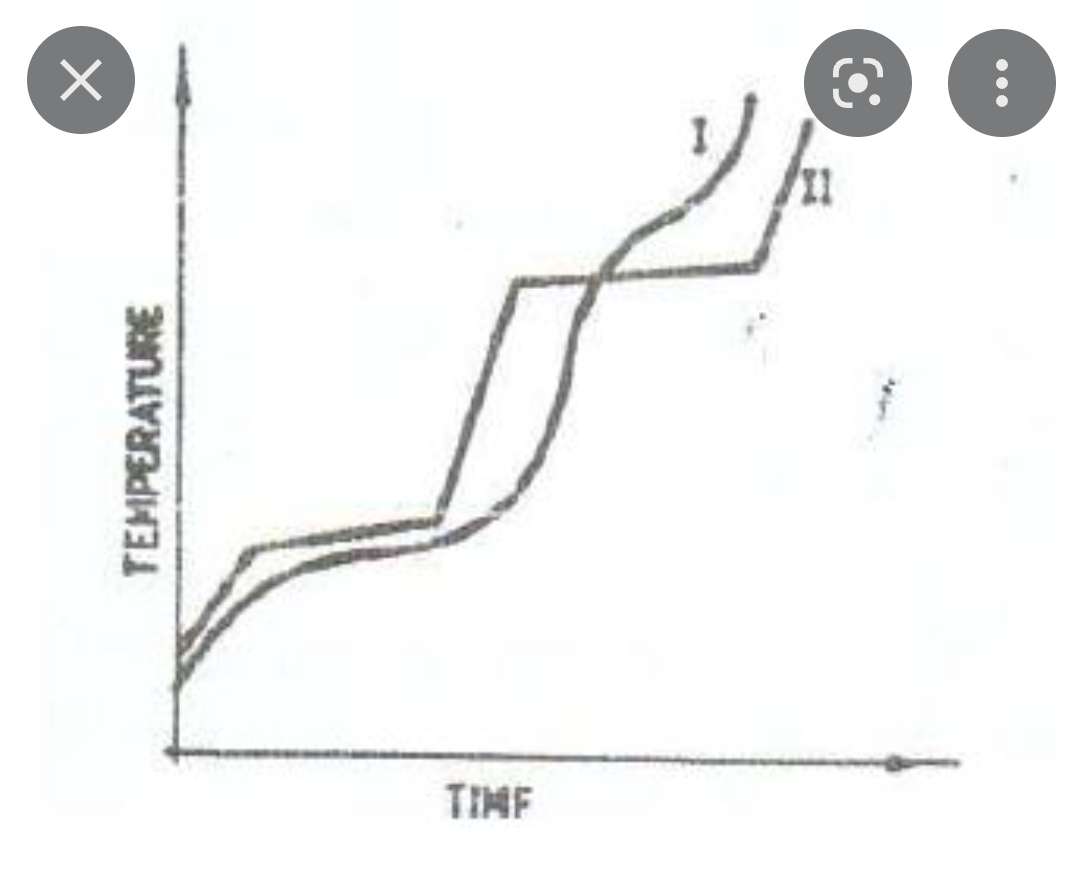
26
New cards
Heating curve of a pure substance
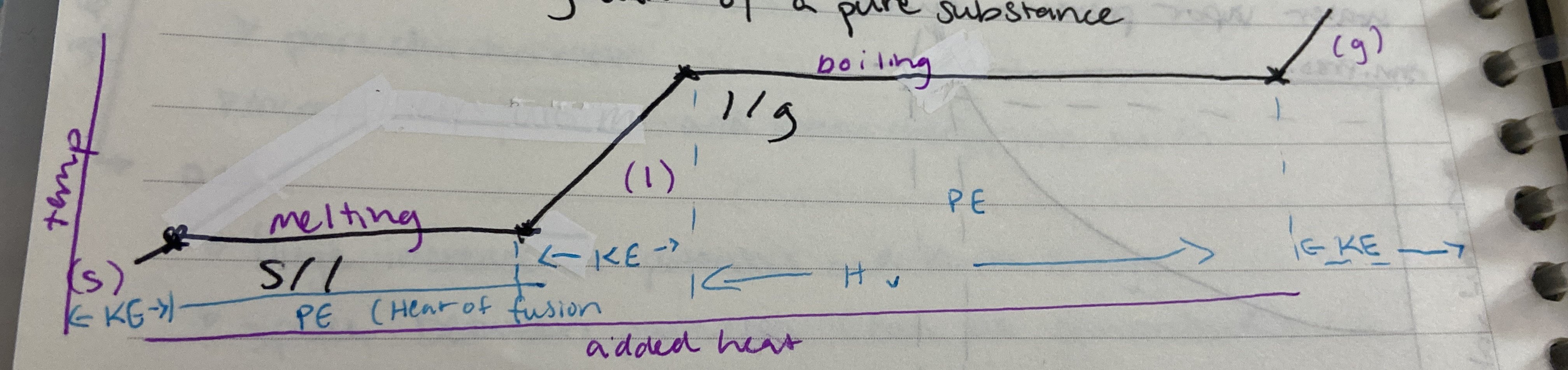
27
New cards
Heating curve of an impure substance (mixture)
constantly increasing KE helps identify purity of a substance
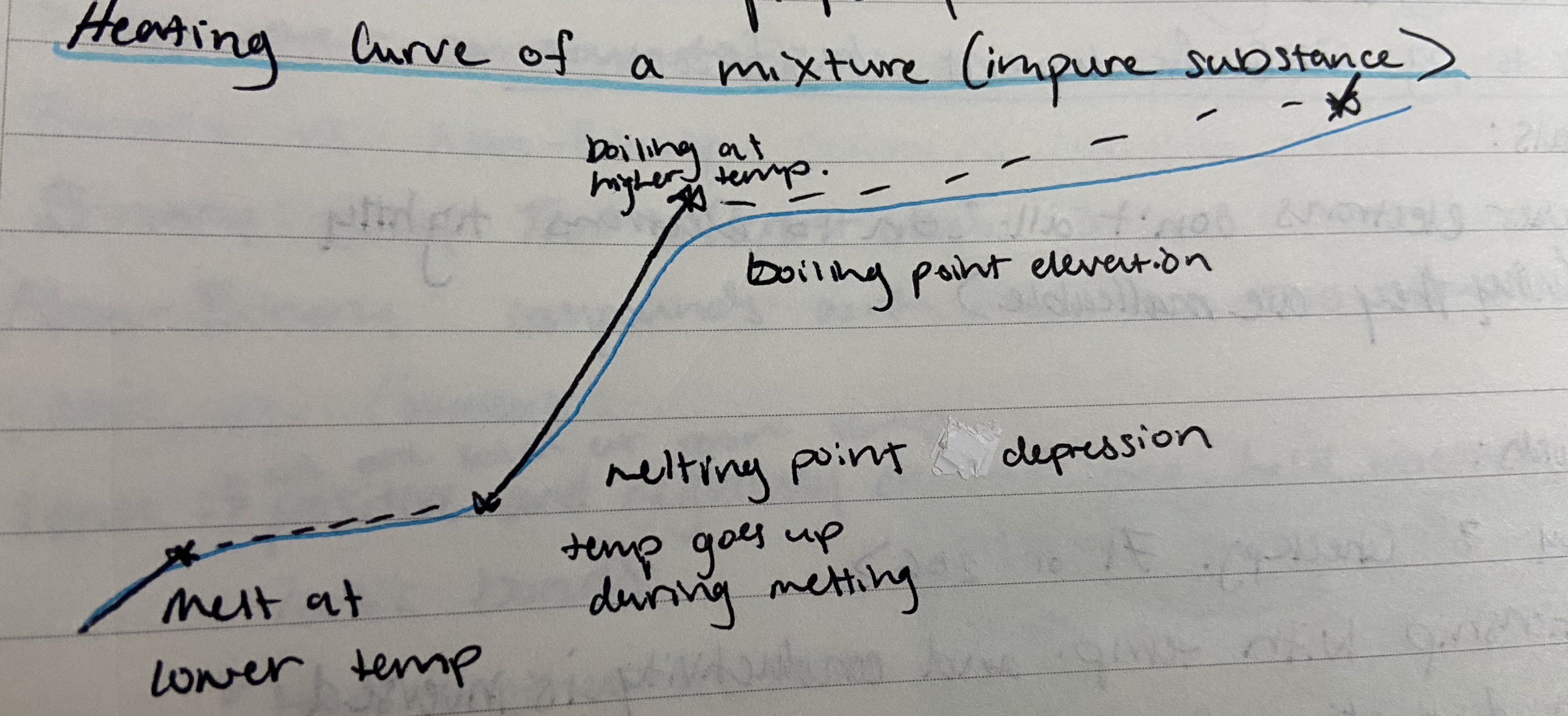
28
New cards
Reactivity
whether a substance reacts; or to a substance's reaction rate
29
New cards
Heat of Formation
the heat released when a compound is formed from its elements (intensive chemical property)
30
New cards
Heat of Combustion
the heat released when a substance undergoes complete combustion (intensive chemical property)
31
New cards
Matter can be further classified as?
Mixtures:
-two or more physically combined substances
-random proportions and properties
OR
Pure substances:
- one type of chemical unit
- chemically combined (amounts can't vary)
- fixed proportions and properties
-two or more physically combined substances
-random proportions and properties
OR
Pure substances:
- one type of chemical unit
- chemically combined (amounts can't vary)
- fixed proportions and properties
32
New cards
Pure substances can be further classified as?
Elements:
- one kind of atom
- cannot be decomposed into other substances
OR
Compounds:
- more than one type of atom chemically combined in fixed proportions
- can be decomposed into constituent elements by breaking chemical bonds
- one kind of atom
- cannot be decomposed into other substances
OR
Compounds:
- more than one type of atom chemically combined in fixed proportions
- can be decomposed into constituent elements by breaking chemical bonds
33
New cards
Elements can be further classified as?
Metals:
- good conductors of heat and electricity
- malleable
- ductile
- lustrious
- outer electrons don't hold on to electrons tightly
OR
Metalloids:
- exhibit some properties of both metals and non-metals
- only 7 of them; most well-known is silicon
- MAIN DISTINCTION is that their relationship with temp and conductivity is inversed
OR
Non-metals:
- poor conductors of heat and electricity
- mostly gases at room temp
- solid crystals are brittle
- good conductors of heat and electricity
- malleable
- ductile
- lustrious
- outer electrons don't hold on to electrons tightly
OR
Metalloids:
- exhibit some properties of both metals and non-metals
- only 7 of them; most well-known is silicon
- MAIN DISTINCTION is that their relationship with temp and conductivity is inversed
OR
Non-metals:
- poor conductors of heat and electricity
- mostly gases at room temp
- solid crystals are brittle
34
New cards
Compounds can be further classified as?
Organic vs Inorganic
Binary vs Non-binary
Ionic vs Covalent
Acids vs Bases vs Salts (some)
Binary vs Non-binary
Ionic vs Covalent
Acids vs Bases vs Salts (some)
35
New cards
Organic vs Inorganic compounds
Organic: most contain carbon and hydrogen atoms bonded together
Inorganic: any other compound
Inorganic: any other compound
36
New cards
Binary vs Non-binary compounds
Binary: only TWO different elements
Non-binary: compounds with more than two elements
Non-binary: compounds with more than two elements
37
New cards
Ionic vs Covalent compounds
Ionic: In ionic compounds, no neutral, independent molecules exist. The formulas that we write for ionic compounds simply represent the smallest whole-number ratios (integral ratio) of cations to anions that are electrically neutral.
- all are solid at room temp
- cations and anions held together in 3D crystal lattices by ionic bonds
- formula is a ratio, not a molecule
- 3D ionic bonds make them very stable
- compounds with alkali metals or polyatomic ions are always IONIC
OR
Covalent:
- formulas represent numbers of atoms that exist in the molecule (it is a ratio)
- most are molecular compounds
(made stable by sharing electrons (covalent bonds)
- all are solid at room temp
- cations and anions held together in 3D crystal lattices by ionic bonds
- formula is a ratio, not a molecule
- 3D ionic bonds make them very stable
- compounds with alkali metals or polyatomic ions are always IONIC
OR
Covalent:
- formulas represent numbers of atoms that exist in the molecule (it is a ratio)
- most are molecular compounds
(made stable by sharing electrons (covalent bonds)
38
New cards
Acids vs Bases vs Salts
Acid: a compound that either starts with H or ends with COOH
Base: contains a hydroxide (OH)
Salt: Any other ionic compound
Base: contains a hydroxide (OH)
Salt: Any other ionic compound
39
New cards
Mixtures can be further classified as?
Homogenous: uniform throughout
OR
Heterogenous: non-uniform
OR
Heterogenous: non-uniform
40
New cards
Homogenous mixtures can be further classifed as?
Solutions:
- the particles in a solution are smaller than the wavelength of visible light
- most common that we see: aqueous solutions (smth dissolved in water)
- solute(s) [minor component] dissolved in a solvent [major component]
- PHYSICALLY COMBINED (properties of all components remain and combine)
- can be in any state
OR
Colloids:
- dispersed phase dissolved in a medium
- translucent and cloudy
- particles are about the same size or big enough to reflect visible light
- display Tyndall effect (shine bright light and it goes through the mixture)
- particles do not settle when standing (can be separated in a centrifuge)
- the particles in a solution are smaller than the wavelength of visible light
- most common that we see: aqueous solutions (smth dissolved in water)
- solute(s) [minor component] dissolved in a solvent [major component]
- PHYSICALLY COMBINED (properties of all components remain and combine)
- can be in any state
OR
Colloids:
- dispersed phase dissolved in a medium
- translucent and cloudy
- particles are about the same size or big enough to reflect visible light
- display Tyndall effect (shine bright light and it goes through the mixture)
- particles do not settle when standing (can be separated in a centrifuge)
41
New cards
Heterogenous mixtures can be further classified as?
Suspensions:
-particles settle upon standing (sediments)
- displays Tyndall Effect
- dispersed phase particles are bigger than 1 micrometre
OR
Mechanical mixtures;
- separated by mechanical means
- includes some mixtures in every class
-particles settle upon standing (sediments)
- displays Tyndall Effect
- dispersed phase particles are bigger than 1 micrometre
OR
Mechanical mixtures;
- separated by mechanical means
- includes some mixtures in every class
42
New cards
What does physical separations of a mixture allow for?
It allows for the constituent substances to be identified. The substances can then be used for their individual properties, values, or recombined into other useful mixtures.
43
New cards
What is the trick to separating substances in a mixture?
Pick a property that clearly differentiates the substances.
44
New cards
What are the 7 separation methods?
1. Solvent extraction
2. Recrystallization
3. Filter separation/decanting
4. Evaporation to dryness
5. Centrifugation
6. Chromatography
7. Simple distillation
2. Recrystallization
3. Filter separation/decanting
4. Evaporation to dryness
5. Centrifugation
6. Chromatography
7. Simple distillation
45
New cards
What is solvent extraction?
The separation of a particular substance from a mixture by dissolving that substance in a solvent that will dissolve it, but which will not dissolve any other substance in the mixture. The most dense substances will end up on the bottom and the least dense will end up on the top. The substance on the bottom is then separated by a funnel.
46
New cards
What does solvent extraction take advantage of?
takes advantage of DIFFERENCES IN SOLUBILITY
- used for separating liquid in liquid mixtures
- used for separating liquid in liquid mixtures
47
New cards
What is recrystallization?
Recrystallization involves dissolving the impure compound (the solute) in an appropriate solvent. The solvent is then heated to allow the solute to better dissolve in the solvent. As the solvent cools, the solution becomes saturated with the solute and the sample with the highest concentration crystallizes out (reforms a solid). The slower it recrystallizes, the more pure the compound was. It should not evaporate to dryness.
48
New cards
What does recrystallization take advantage of?
TAKES ADVANTAGE OF DIFFERENCES IN CONCENTRATION
- used for a large concentration of one solid mixed with a small concentration of another solid
- used for a large concentration of one solid mixed with a small concentration of another solid
49
New cards
What is filtration?
A separation technique used to separate the components of a mixture containing an undissolved solid in a liquid. The liquid containing the sediment is poured into a folded piece of filter paper in a funnel. The substance stuck in the filter is the residue. The substance the funnelled out of the filter is the filtrate.
50
New cards
What does filtration take advantage of?
TAKES ADVANTAGE OF DIFFERENT SIZES OF PARTICLES
51
New cards
What is decanting?
The process of separating a liquid from a solid and other immiscible (non-mixing) liquids, by removing the liquid layer at the top from the layer of solid or liquid below. The process can be carried out by tilting the mixture and pouring out the top layer, while taking care so the bottom layers do not pour out as well. There will be a little bit of the top liquid left in, but you can heat up the solids to dry off the liquid and keep a more precise amount of the solids.
52
New cards
What does decanting take advantage of?
TAKES ADVANTAGE OF DIFFERENT SIZES OF PARTICLES
53
New cards
What is evaporation to dryness?
A process where a mixture of a solid dissolved in a liquid is left to evaporate to dryness. The solid will then be left behind as a residue. It is done by evaporating the solvent off, measuring the mass, and then measuring the concentration of the solution. This is used when we do not care about recovering the solvent.
54
New cards
What does evaporation to dryness take advantage of?
TAKES ADVANTAGE OF BOILING POINTS
55
New cards
What is centrifugation?
A method of separating suspended particles in a colloid or suspension and causing them to sink or rise depending on their density by spinning them at a high speed. Particles in a centrifuge continue to travel in a straight line while the centrifuge tube turns. The densest particles with the greatest inertia are selectively directed to the bottom of the tube.
56
New cards
What does centrifugation take advantage of?
TAKES ADVANTAGE OF DIFFERENT DENSITIES
57
New cards
What is the density of water?
1.0 g/mL
58
New cards
What is chromatography?
A technique for separating mixtures into their constituent parts. At its core, chromatography separates substances in a solution by having a mobile phase (which carries the mixture being separated) carry them at different rates through a stationary phase (which the solutes adhere to). Each substance travels through the stationary phase at its own characteristic rate according to how well it dissolves in the mobile phase and how well it adheres to the stationary phase. Cannot use this on clear substances.
59
New cards
What does chromatography take advantage of?
TAKES ADVANTAGE OF SOLUBILITY IN THE MOBILE PHASE AND ADHESION TO THE STATIONARY PHASE
60
New cards
What is capillary action?
The tendency of a liquid to rise in narrow tubes or to be drawn to small openings. It causes the solvent to rise up through the stationary medium in chromatography.
61
New cards
Formula for ratio of fronts
solute front/solvent front
62
New cards
What does the ratio of fronts help us do?
Identify or support the identification of a substance by more definitive means.
63
New cards
Formula for separation distance
spn. distance = d2 (Rfa - Rfb)
64
New cards
What is simple distillation?
A method of separating dissolved solids from a liquid solvent based on differences in their volatilities in a boiling liquid mixture. The components in a sample mixture are vaporized by the application of heat and then immediately cooled and collected by the action of cold water in a condenser. This is used when we care about recovering the solvent.
65
New cards
What does simple distillation take advantage of?
TAKES ADVANTAGE OF BOILING POINTS/VOLATILITIES
66
New cards
What happens when there are mobile ions dissolved in a solvent?
Mobile ions are a conductive system, so the mixture should conduct electricity.
67
New cards
What do formulas of ionic compounds represent?
The smallest whole-number ratios of cations to anions that are electrically neutral (formula unit)
68
New cards
What compounds are for sure ionic?
Any compound containing the alkali metals or polyatomic ions.
69
New cards
How can we find the charge of a multivalent metal ion from its name?
The roman numeral indicates the charge
70
New cards
If we know the charge of one ion, how do we know the charges of the rest of the ions in that family?
Families usually have the same charges; base your charges off of the table of ions and cope i guess
71
New cards
Do ionic compounds use prefixes?
No
72
New cards
Why do we need to be careful when writing formulas with polyatomic ions?
We need to be careful with the brackets
73
New cards
Do covalent compounds need prefixes?
Yes. (memorize them ;-;)
74
New cards
What are the prefixes used for covalent compounds?
1 - mono
2 - di
3 - tri
4 - tetra
5 - penta
6 - hexa
7 - hepta
8 - octa
9 - nona
10 - deca
2 - di
3 - tri
4 - tetra
5 - penta
6 - hexa
7 - hepta
8 - octa
9 - nona
10 - deca
75
New cards
What are hydrates?
Salts where water locks itself into the pre-existing crystal lattice.
76
New cards
What is an anhydrous salt?
A salt without water locked into it. (form of the salt without water)
77
New cards
What are hygroscopic salts?
Salts that absorb water out of the air to form hydrates.
78
New cards
What are dessicants?
Hygroscopic salts that keep containers dry
79
New cards
Do hydrates use prefixes?
Only for the hydrate portion of the name
80
New cards
How do we name binary acids?
Hydro- -ic acid
81
New cards
What are oxyacids?
Three element acids made up of hydrogen, oxygen, and something else
82
New cards
How do we name oxyacids ending in "-ate"?
-ic acid
83
New cards
How do we name oxyacids ending in "-ite"?
-ous acid