BS132 Lecture 1
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Describe Protons
1u mass, positive, 1.67×10^-27kg
Describe Electrons
Negligible mass, negative, 9.11×10^-31kg
Describe Neutrons
1u mass, no charge
How does the mass of electrons relate to the mass of protons?
1 e(-) = 1/2000 x p(+)
What is Mass Number?
Protons + Neutrons
What is Atomic Number?
Number of Protons or Electrons
What does a Lewis Diagram show?
Valence electrons only
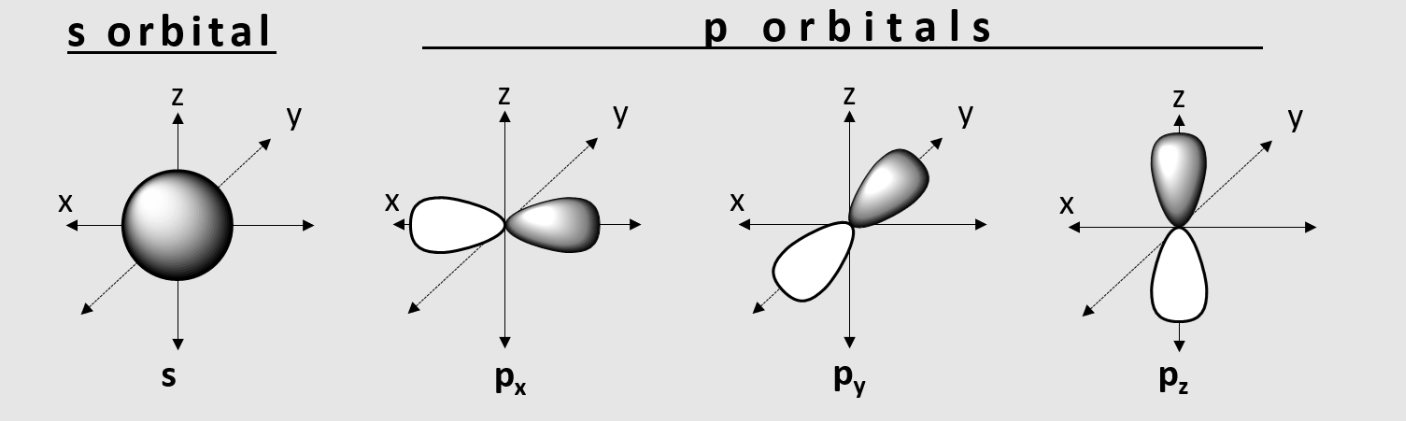
What are Atomic Orbitals?
3D areas w/in which electrons may be
Types of s and p orbitals
s, Px, Py, Pz
What is the Aufbau Principle?
Electrons enter the lowest energy orbital that’s available
What does Degenerate mean?
Equal in energy
What is Pauli’s Exclusion Principle?
Orbitals accomodate a max of 2 electrons and they must have opposite spins (spin paired)
What is Hund’s Rule of Max Multiplicity?
Lowest energy configuration for a partially occupied set of degenerate orbitals has as many e(-)s as possible residing in different orbitals w/ their spins in same direction