Chapter 10: Ideal gas laws
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
STP (standard temp. and pressure)
273.15K and 1 atm
Combined gas law
(P1V1)/T1 = (P2V2)/T2
Ideal gas law
PV = nRT
R constant =
8.314 J/mol
Equation for formula weight using ideal gas law
FW = (Density x R x T)/P
Dalton’s Law
(Pa)/Ptotal = (na)/ntotal
Graham’s Law

Properties of gas particles
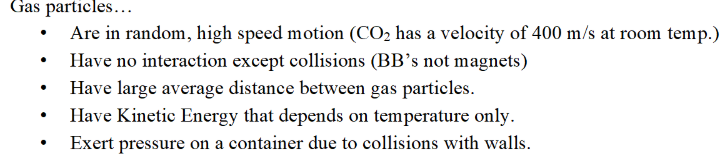
How does the pressure of a real gas compare to the pressure of an ideal gas at low temperatures?
At low temperatures, the pressure of a real gas is lower than the pressure of an ideal gas due to the effect of intermolecular forces affecting the collisions between molecules
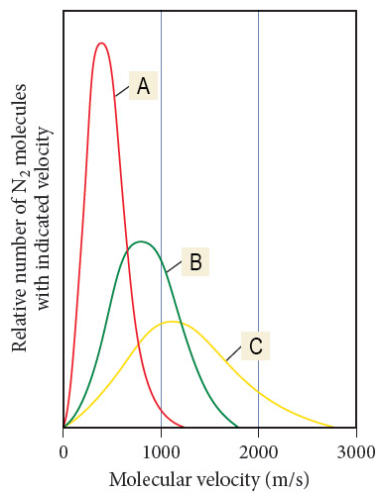
Which letter has the highest temp.?
C
When do gases behave the least like ideal gases?
At low temperatures and high pressures
Pressure and volume are _________ proportional’ whose law?
inversely; Boyle’s
Volume and temperature are _________ proportional; whose law?
directly; Charles’
Temperature and pressure are _________ proportional; whose law?
directly; Gay-lussac’s