Formation of Ions
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
when are ions made?
ions are made when electrons are transferred
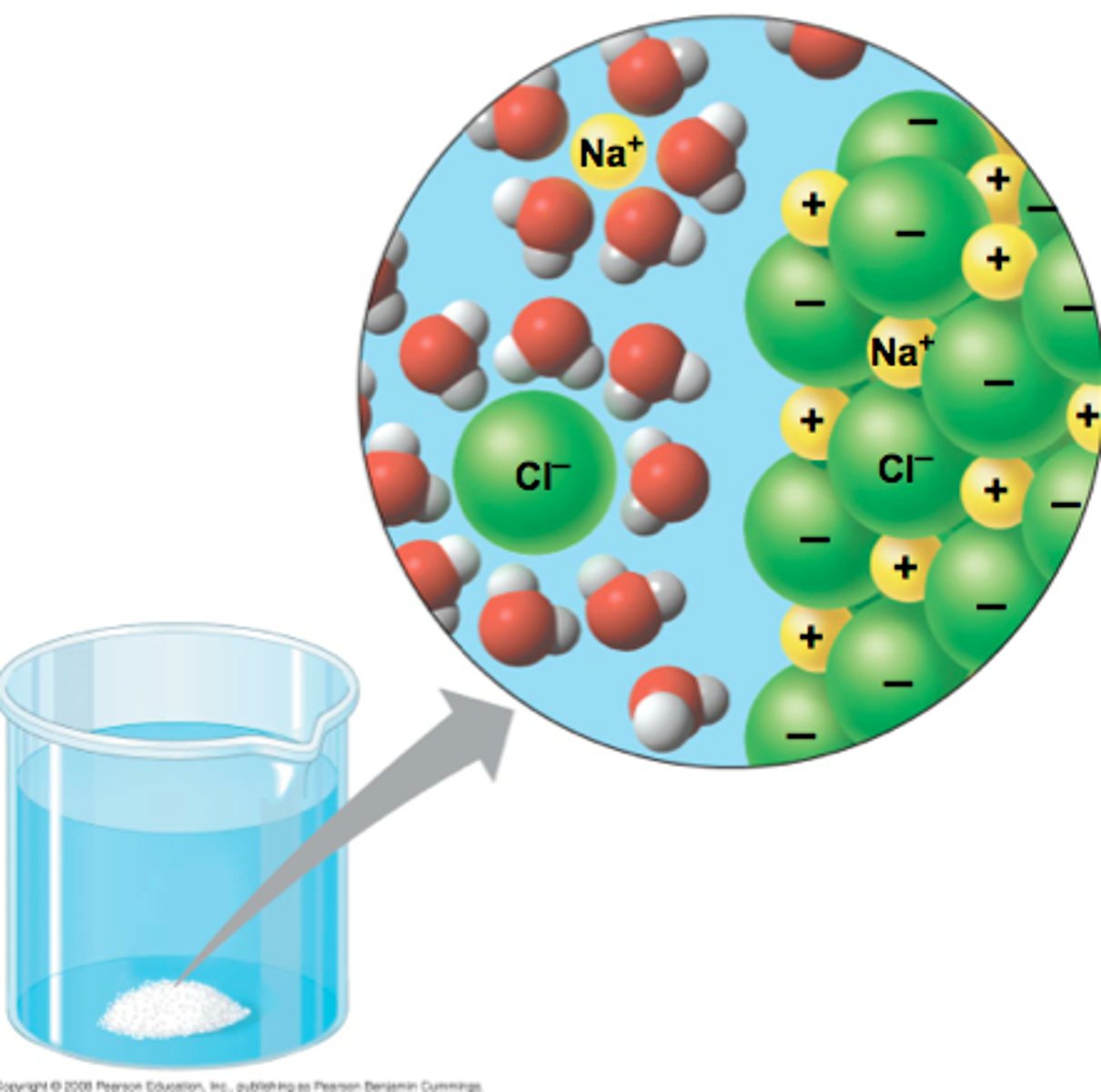
what is an ion?
ions are charged particles, they can be single atoms or groups of atoms
when metals form ions what do they do?
they lose electrons from their outer shell to form positive ions
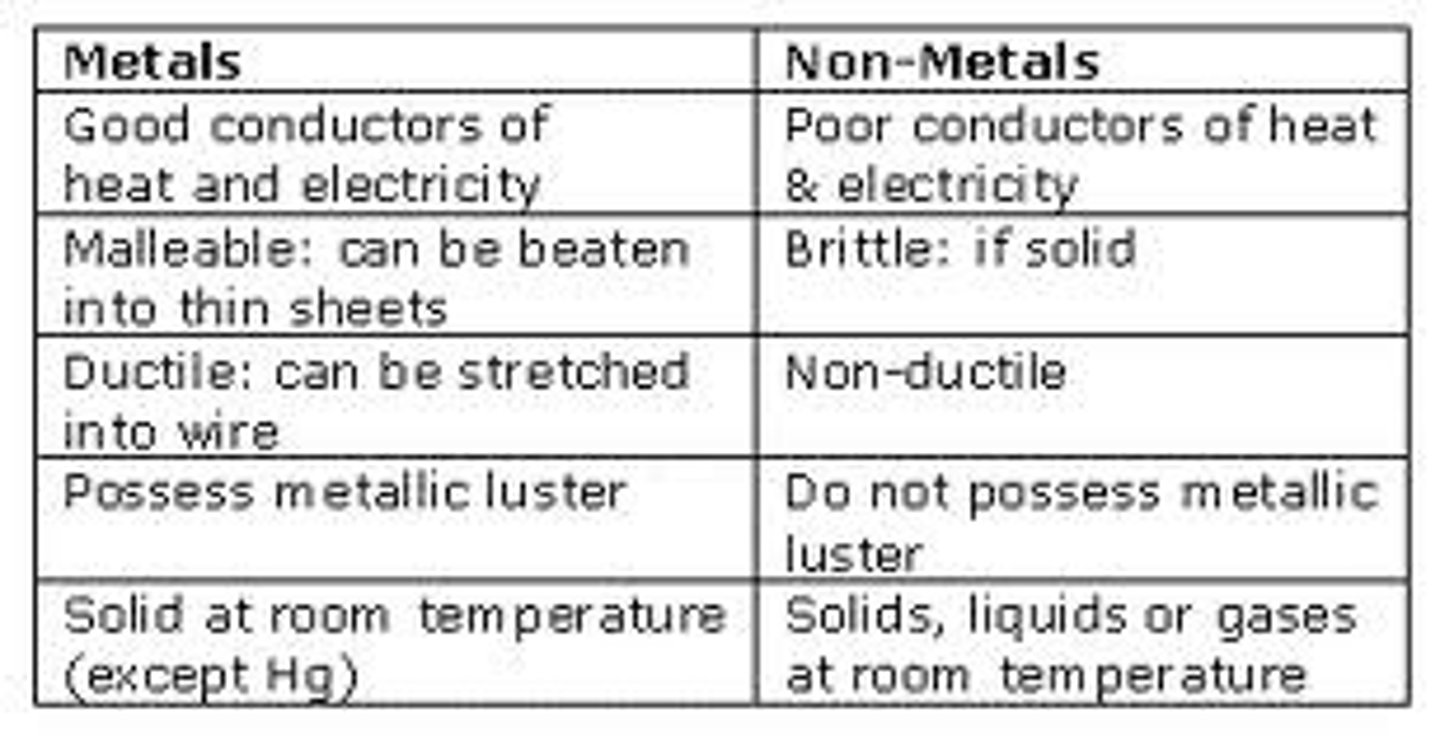
when non-metals form ions what do they do?
they gain electrons into their outer shell to form negative ions
what is the number of electrons lost or gained the same as?
the charge of the ion eg lose 2 is a 2+ charge
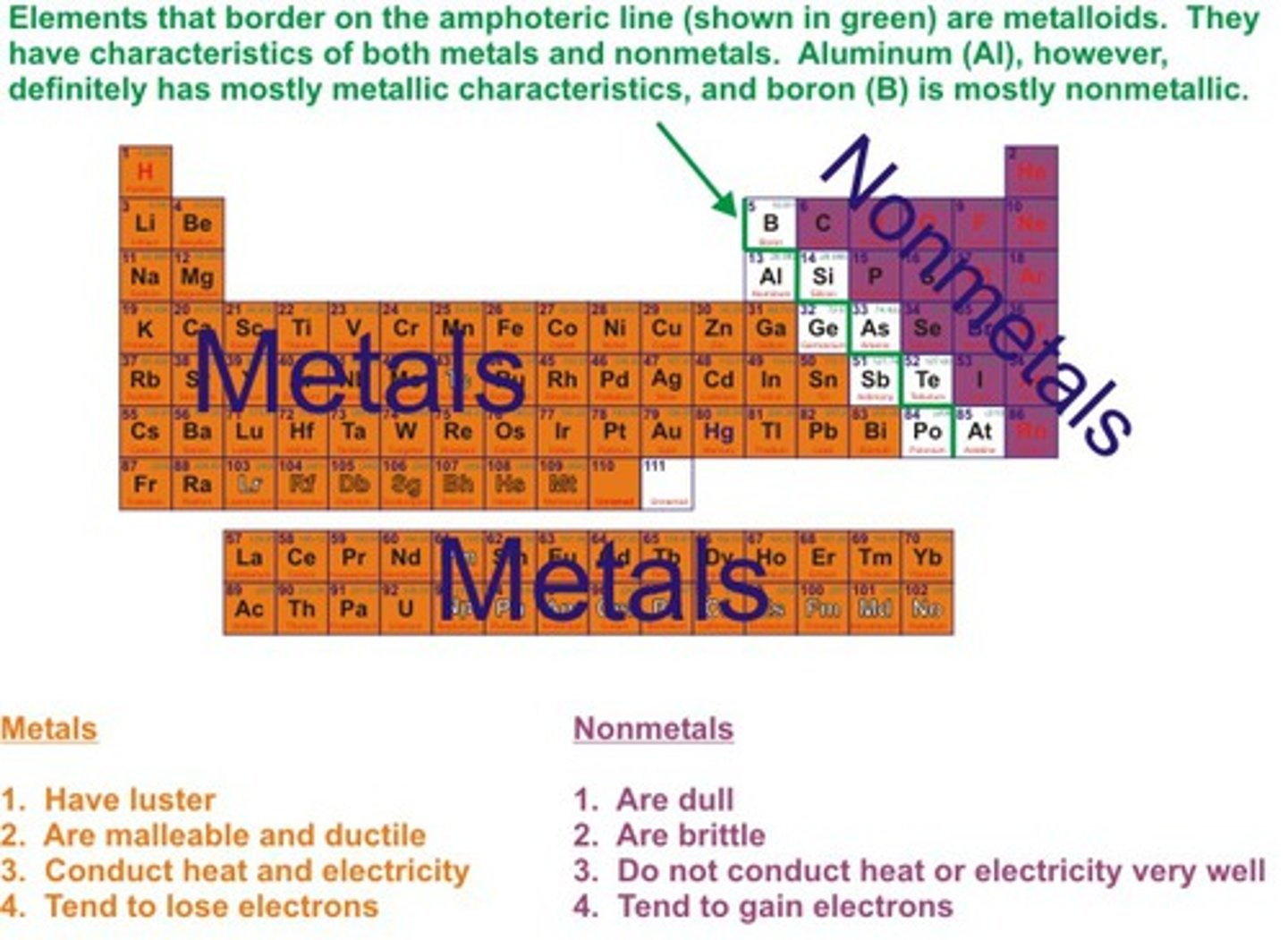
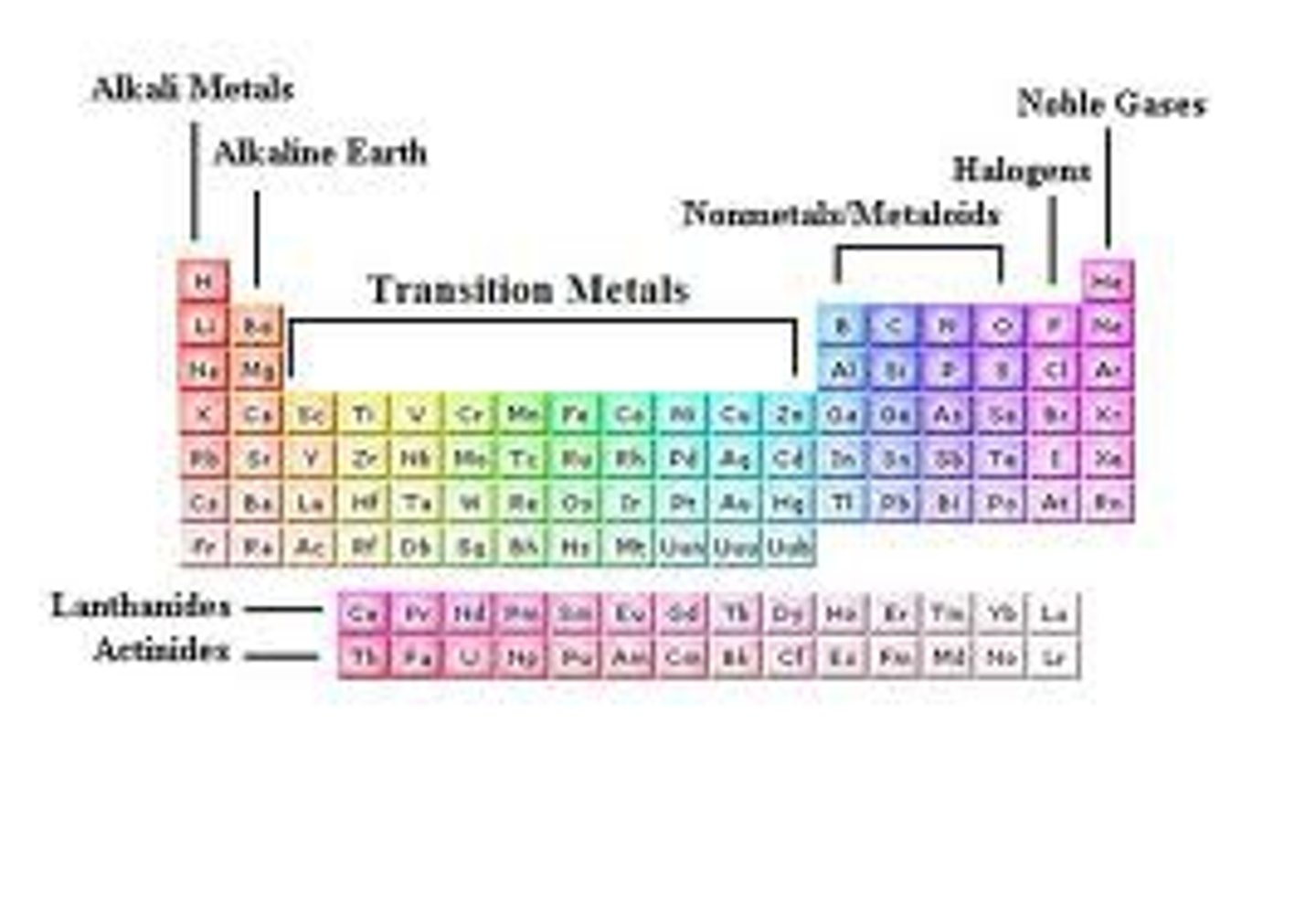
which groups are most likely to form ions?
1, 2, 6 & 7
what type of elements are groups 1 & 2
metals
how do groups 1 & 2 form ions?
they lose electrons to form positive ions
what type of elements are groups 6 & 7?
non-metals
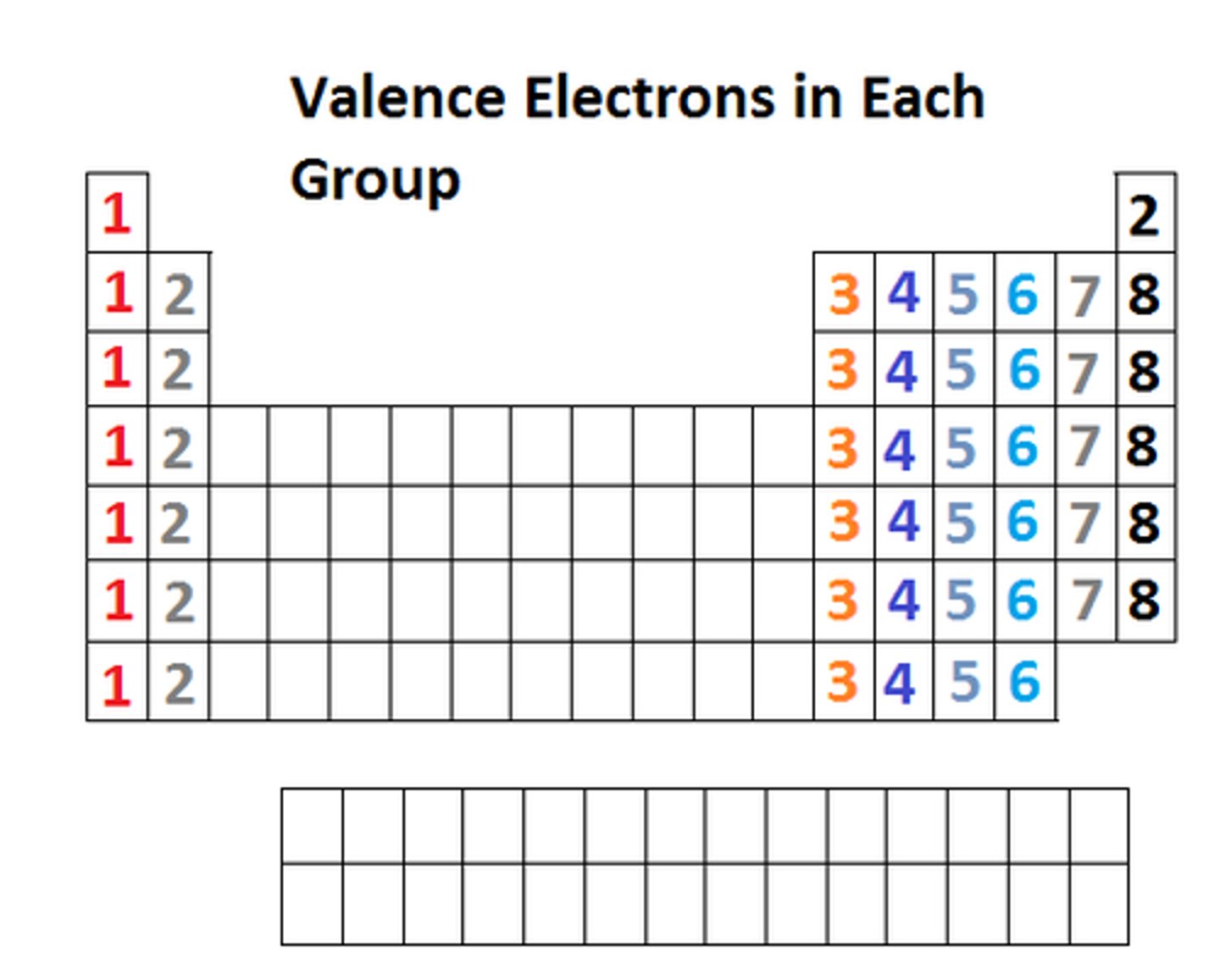
how do groups 6 & 7 form ions?
they gain electrons to form negative ions
are cations positive or negative?
positive

are anions positive or negative?
negative
what do elements in the same group have the same number of?
outer electrons
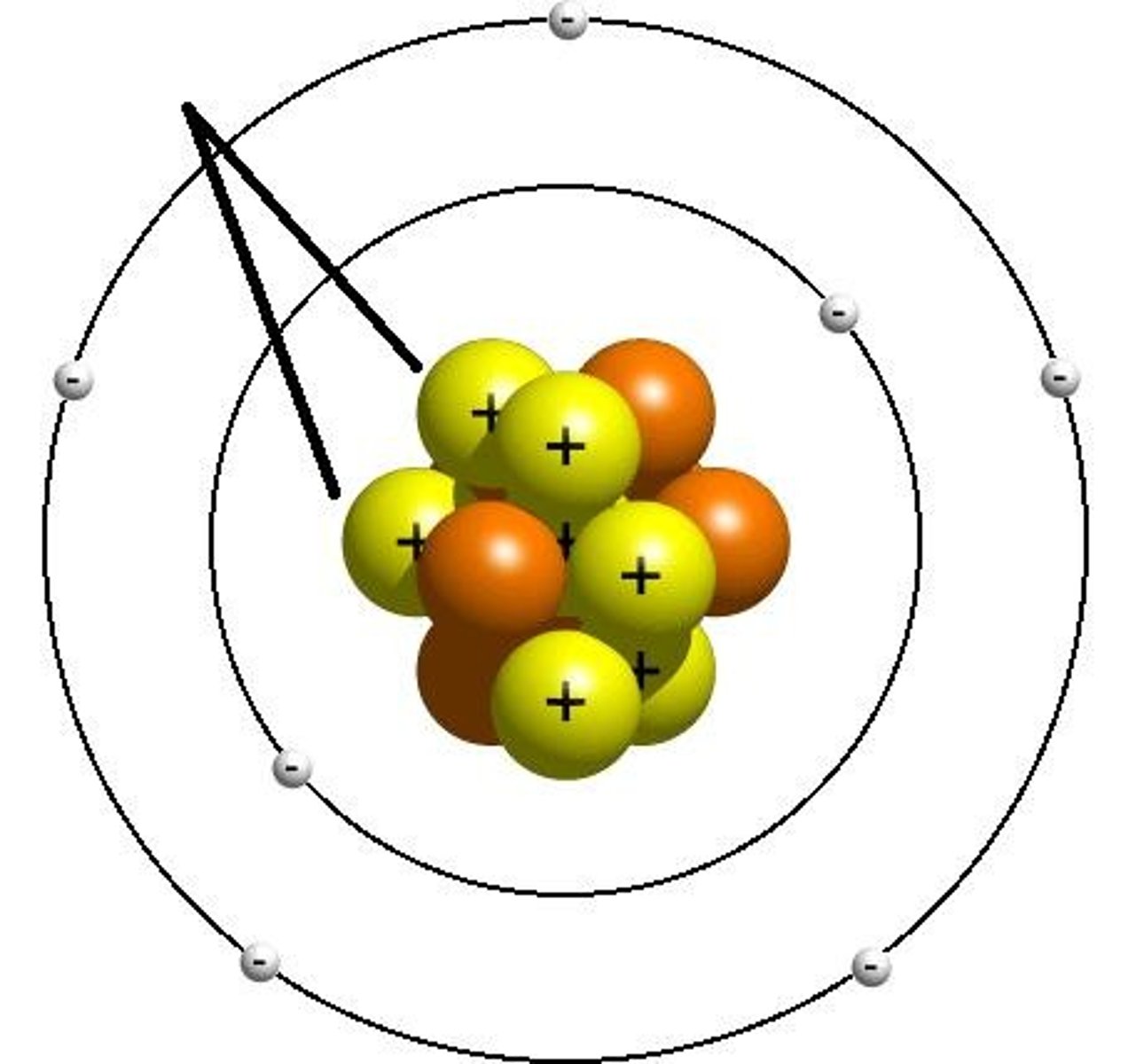
what charge ions does group 1 form?
1+
what charge ions does group 2 form?
2+
what charge ions does group 6 form?
2-
what charge ions does group 7 form?
1-
how does sodium form an ion?
loses 1 electron
what's the electron equation for sodium?
Na goes to Na+ + e-
which group is magnesium in?
group 2
how does magnesium form an ion?
loses 2 electrons
what's the electron equation for magnesium?
Mg goes to Mg2+ + 2e-
what group is chlorine in?
group 7
how does chlorine form an ion?
gains 1 electron
what's the electron equation for chlorine?
Cl + e- goes to Cl-
which group is oxygen in?
group 6
how does oxygen form an ion?
gains 2 electrons
what's the electron equation for oxygen?
O + 2e- goes to O2-