DPT
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Uses of IR
limited amount of sample, for rapid analysis, identifying absorbed compounds on glass and other surfaces, chemical functionalities of fibers, probing compositions of polymer films
higher because it does not have a monochromator and all wavelengths of light hit the detector at once making the signal much higher than the noise “multiplex advantage” The lamp power is also very bright compared to the detector noise because all wavelengths are striking simultaneously
does FTIR detect higher or lower absorbances than UV-Vis
6 absorbance unit
limit of linearity for FTIR
effects the chemical and mechanical properties of the polymer
what does the R group do on a polymer R=-CH=CH2
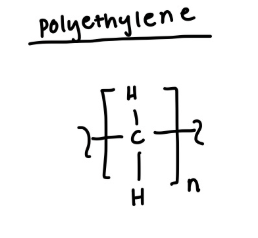
polyethylene
used for sandwich bags
polypropylene
used for soda bottles
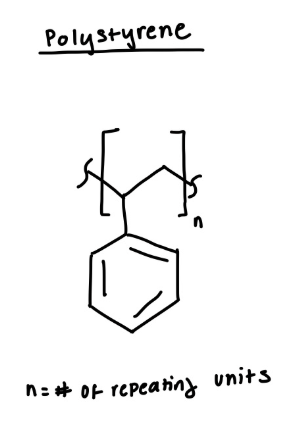
polystyrene
coffee cups
polyvinyl chloride (pvc)
used for food wraps and piping
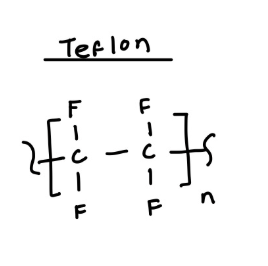
teflon (polytetrafluoroethylene)
material for making non-stick pans
additives
added to affect flammability and pliability of polymers
remove the phthalate added as plasticizer and make Tygon plia
goal of this lab
name and structure of the plasticizer
Bis(2-ethylhexyl) phthalate
2800 to 3000 cm-1
range to expect methylene stretches
structure of polystyrene
structure of polyethylene
structure of Teflon
acetone
the solvent used to extract the plasticizer
the polymer became more malleable and transleucent
effect of the plasticizer removal
Teflon → polystyrene → polyethylene
order for most pliable unknown polymers