MCAT Chemistry
1/217
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
218 Terms
nucleus
where are protons and neutrons located?
where are e- located?
e- cloud
atomic mass
isotopic mass
specific mass of certain isotopes
atomic weight
avg of all masses of naturally occurring isotopes
abundance x atomic mass
atomic number
number of protons
mass number
sum of amt of protons + neutrons in an atom
isotopes always have the same atomic #
principle shell
where e- are
the one closest to nucleus is most stable = ground state
electron shells
the farther the e- is from nucleus, the greater the E
each e- in same shell has same E
# e- in shell = 2n2
spectra
range of wavelengths/frequencies emitted/absorbed
excitation
e- absorbs E + moves to a level that can handle the E
de-excitation
e- releases E as heat/light
color it can reflect depends on element
emission of photon/E = wavelength
energy level
where e- might be, done by determining principal quantum #
principle quantum number
describes shell of atom
high n = high distance from nucleus
subshell
area w/in shell that describes shapes of spaces where e- might be
3D
each E level has subshells
azimuthal quantum numbers (I)
subshell of orbital (shape)
values only range from 0 to (n-1)
magnetic quantum number
s: 1s orbital/orientation
p: 3p orbitals/orientations
d: 5d orbitals/orientations
f: 7f “
electron spin number
orientation which e- spinning
if final e- arrow points up or down
½ = up -½ = down
Aufbau principle
e- fill lowest level first
order: 1s, 2s, 2p, 3s, 3p, 4s, 3d
each e- will hold 2e- w opposite spins
e- will fill up orbitals of same E first
Pauli Exclusion principle
no e- can share same quantum #s
Hund’s rule
when assigning e- it must be individually placed b4 pairing up
ions
elemental atoms that have diff # of e-
isotope
diff # of neutrons
photons
main units of light that has dual particle-wave characteristic
metals
malleable, ↑conductivity bc of willingness to give up e-
have ↓ionization E + ↓e- affinity
can attain multiple oxidations states
good reducing agents
nonmetals
↑ionization E, ↑e- affinity, ↑electronegativity
non-malleable, brittle, soft solids
many are gases @ room temp
metalloids
btwn metals + nonmetals (staircase ones)
semiconductors
ionization energy
E needed to remove e- from a natural gaseous atom
electron affinity
E emitted/absorbed when e'- is added to atom
oxidation states
shows # of e- lost/gained to form chem bond
electronegativity
attraction of other molecules to each other
semiconductor
molecules that can conduct + insulate E
valence electrons
e- in outermost shell + participate in chem bonding
effective nuclear charge
inward/pulling force of pos nuclear charge on valence e-
increases going left to right on periodic table
Zeff = Z — S
S: all e- except valence
1st E level will have greatest nuclear charge
Zeff decreases while principle quantum # increases
bc inner core e- shield some (+) charge
periodic table trends

alkaline earth metals
group 2
↓e- affinity/negativity + ionization E
bc only have 1-2 e- in valence
prone to form cations bc they lose e- easily
moving down, reactivity + atomic density ↑
melting + boiling pnt ↓
Be, Mg, Ca, Sr, Ba, Ra
alkali metals
group 1
soft, ↓density + melting pnts
melting + boiling pnts ↓ going down
reactivity ↑moving down
Li, Na, K, Rb, Cs, Fr
chalcogens
group 16
nonmetals, metalloid + metal
metallic character ↑ as you move down
6 valence e-
O, S, Se, Te, Po, Lv
halogens
groups 17
7 valence e- + readily accept/share an e-
form ionic bonds w alkali + covalent w other nonmetals
going down grp:
melting/boiling pnt ↓
reactivity ↑
F, Cl, Br, I, At, Ts
noble gases
group 18
don’t need to bond w other atoms
inert/inactive
color-less + non-flammable
boiling point ↑
He, Ne, Ar, Kr, Xe, Rn, Og
transition metals
groups 3-12
have diff oxidation states
valence e- in d orbital so Zeff is low so e- move easier
↑melting/boiling points
good heat + electricity conductors
elements found in center need more oxidation states
ionic bonds
transfer of e- from 1 element’s valence shell to another
forms ions + bonds w other ions via electrostatic interactions
bonds constructed via interactions btwn transfer of e-
higher bond strength than covalent
covalent bonds
atoms share e- w one another to form bonds + fulfill octet/duet rule
octet rule
chem bonds form where valence shell of all atoms will have 8e-
mainly applies to s + p orbitals
peroxides and molecules w transition metals don’t follow this
duet rule
only H+ + He
want to have 2e- in valence shell
bonding electrons
2e- shared in bonds
nonbinding electrons
don’t participate in bonding interactions
free e- bc they remain free in orbitals not participating in bonding
formal charges
way to assign hypothetical charges on molecules
formal charge = valence e- — counted e-
formal charge rules
sum of formal charges should always = charge of molecules
neutral molecule = 0
ion = -3
try to have all formal charges = 0
put negative formal charges on more electronegative atom
electron geometry
arrangement of all e- pairs
bonding + nonbonding
molecular geometry
arrangement of bonding e-
Van der Waals
weakest
rely on weak dipole-dipole interactions
occurs when there’s a temporary polarization w/in molecules bc of distribution of e- → generates temporary dipole
hydrophobic + nonpolar molecules only do this one
dipole-dipole interactions
permanent dipoles formed
bc of differences in distribution of e-
↑electroneg = ↑e- density
more electronegative atom has (-) dipole + other one has (+) dipole
Hydrogen Bonding
strongest of 3
H-bond donor + H-bond acceptor
donor = electroneg atom w H
acceptor = electroneg atom
occur w O,F,N
molecular weight
sum of all individual atoms in molecule
mole
6.022×1023 = 1mole
molar mass
mass in grams of 1 mole of substance
# of moles x molar mass
g/mol
molecular formula
elemental composition of compound
empirical formula
ratio btwn all atoms in 1 compound (simplifying)
synthesis reaction
combine 2+ reactants to form product
2H2 + O2 → 2H2O
decomposition reaction
breaking down reactant to simplest form
2NaCl → 2NA + Cl2
combustion reaction
substance (usually hydrocarbon) reacts w O to produce heat, CO2 + water as byproduct
CH4 + 2O2 → CO2 + 2H2O + heat
single displacement reaction
displacement of reactant to form new product
Zn + 2HCl → H2 + ZnCl2
double displacement reaction
exchange of ions in compound to form new product
NA2SO4 + SrCl2 → 2NaCl + SrSO4
law of conservation of mass
in chem rxn, matter cannot be created/destroyed
balancing equations so #reactants = #products
activation energy
energy needed for rxn to proceed
rate determining step
slow step
determines rate of rxn
catalyst
increase rate of rxn
consumed 1st + produced later
don’t show in equation bc they’re reproduced
intermediates
chem species formed in 1 step of rxn + consumed in next
don’t show in equation
multistep chemical reaction
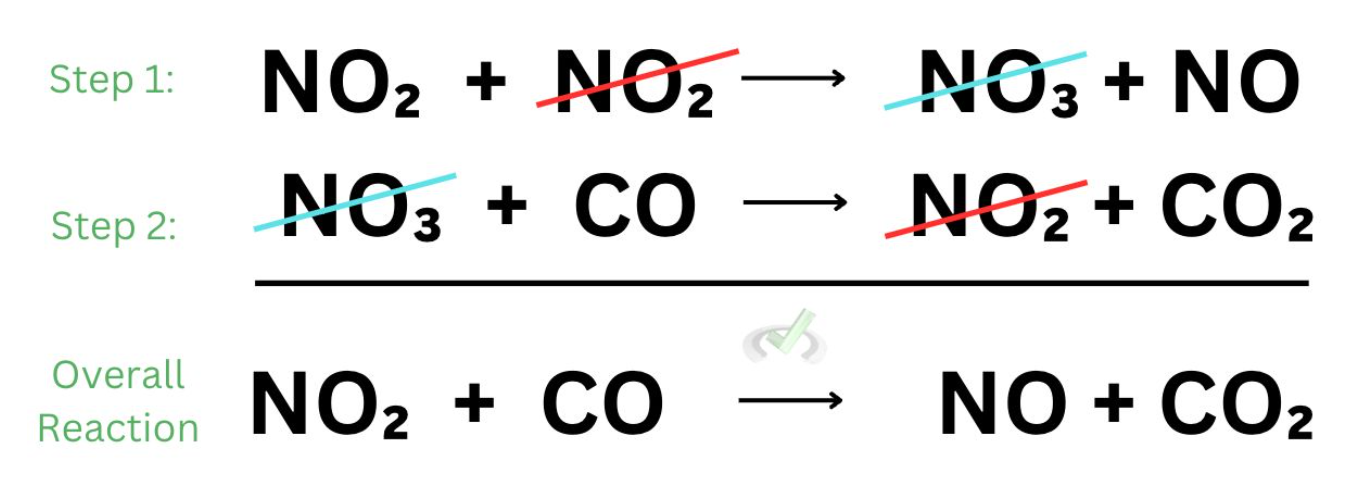
collision theory
examine collisions btwn reactants that form products
↑temp = ↑collision = ↑products
↑[reactants] = ↑collision = ↑products
transition state theory
↑activation E → ↓rxn rate
(the humps on energy graph are the states)
reaction rate
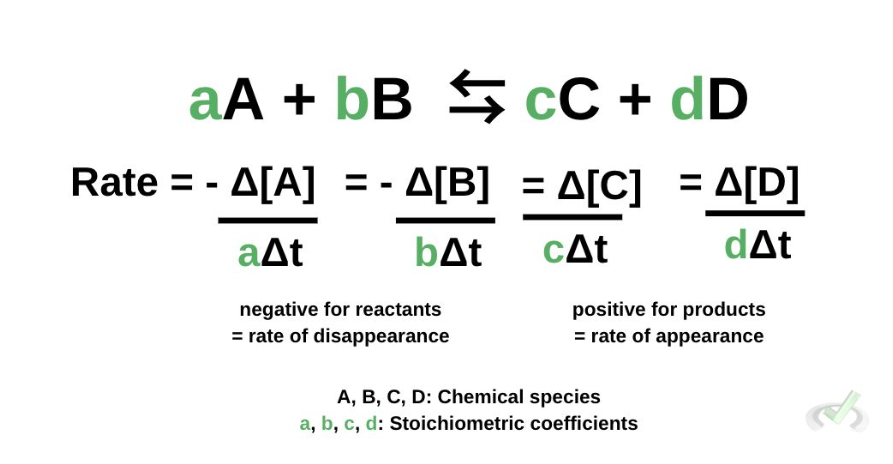
rate law
Rate = k[A]x[B]y
![<p>Rate = k[A]<sup>x</sup>[B]<sup>y</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/f8e44bbc-7c24-4b0c-a7e5-ed70725947e8.png)
finding constant “k” in rate law
either [A] or [B] cancels out
![<p>either [A] or [B] cancels out</p>](https://knowt-user-attachments.s3.amazonaws.com/73e185cb-d163-4880-bbf3-c4c81196dfde.png)
dynamic equilibrium
reactants turn to products + products to reactant @ same rate
concentrations remain constant
equilibrium constant
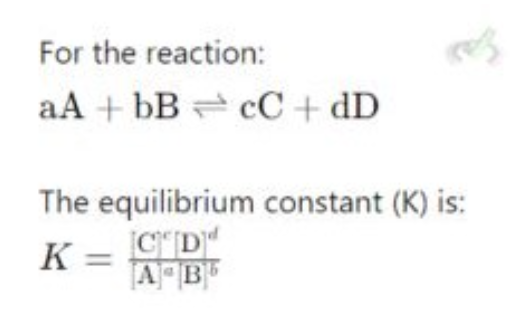
Le Chatelier’s principle
if system @ equilibrium is disturbed, system will adjust to counteract
concentration
pressure
temp (exothermic = ↑temp = ↑reactants)
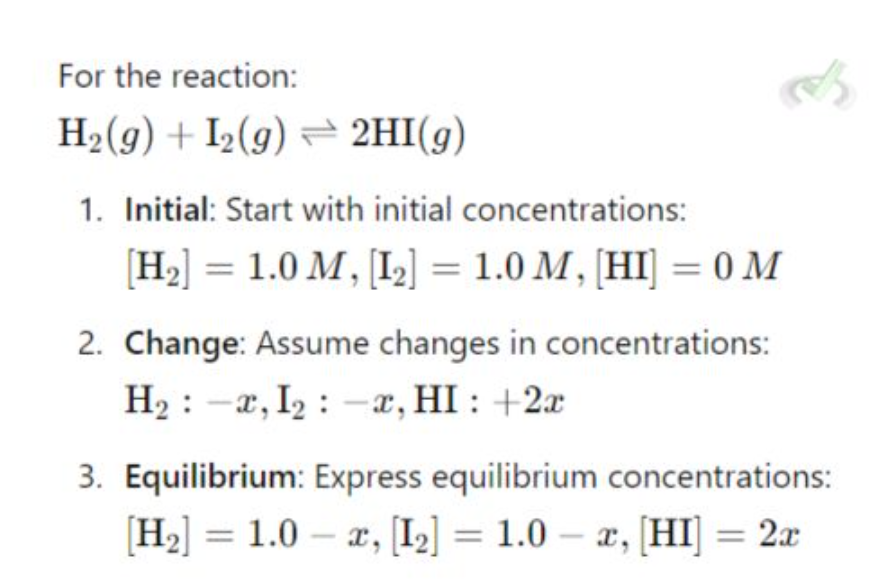
ICE Table (initial, change, equilibrium
to find equilibrium concentrations
Haber process
agriculture
produces ammonia for fertilizers
N2(g) + 3H2 ←→ 2NH3(g)
contact process
produces sulfuric acid
2SO2(g) + O2(g) ←→ 2SO3(g)
kinetic product
form faster
↓Ea needed
favored at ↓temp
less stable
thermodynamic product
more stable
takes longer
favored @ ↑temp
needs ↑Ea
Diels-Alder Reaction
diene + dienophile = kinetic + thermodynamic product
enolate formation
kinetic: fast + uses strong, bulky base (LDA)
thermodynamic: @ ↑temp + uses smaller base → ↑stable enolate
system (thermodynamics)
environment/universe being studied
surrounding (thermodynamics)
everything outside system
open system
can exchange E + matter w surrounding
closed system
can exchange only E w surrounding
isolated system
cannot exchange anything
state functions (thermodynamics)
depends only on state of system, not how it got there
ex: temp, P, V
internal energy (U)
total E w/in system
all kinetic + potential E of particles
ΔU = q + w
q = heat w = work
enthalpy (H)
heat content of a system @ constant P
heat absorbed/released @ constant temp
ΔH = ΔU + PΔV
ΔH = ∑Hproducts + ∑Hreactants
-ΔH = exothermic
entropy (S)
disorder/randomness of system
ΔS = qrev/T
high S = high disorder
gas has high entropy
ΔS = ∑Sproducts + ∑Sreactants
Gibbs free E (G)
determines if rxn if spontaneous
ΔG = ΔH - TΔS
ΔG<0: spontaneous
ΔG>0: non-spontaneous
ΔG=0: equilibrium
isothermal process
occurs @ constant temp
ΔU = 0
adiabatic process
occurs w/o heat exchange
all E Δs come from work done on/by system
isochoric process
occurs @ constant V
work = 0
added heat changes U
isobaric process
occurs @ constant P
heat added/removed Δs H
heat capacity (C)
amt of heat needed to Δ temp of substance by 1oC
C = q/ΔT
standard enthalpy of formation (ΔHfo)
Δq when 1 mole of compound is formed from its elements in standard states