B) elements, compounds and mixtures (copy)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
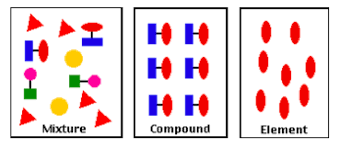
element definition
substance that can’t be split into anything simpler by chemical means
contains only one type of atom
e.g. oxygen gas, a pure metal (like Mg), diamond
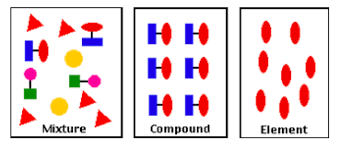
compound definition
formed when 2 or more elements chemically combine
e.g. water, sodium chloride
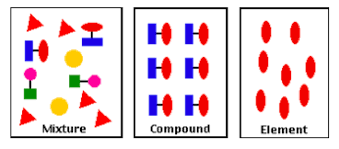
mixture definition
various substances mixed together + no chemical reaction occurs
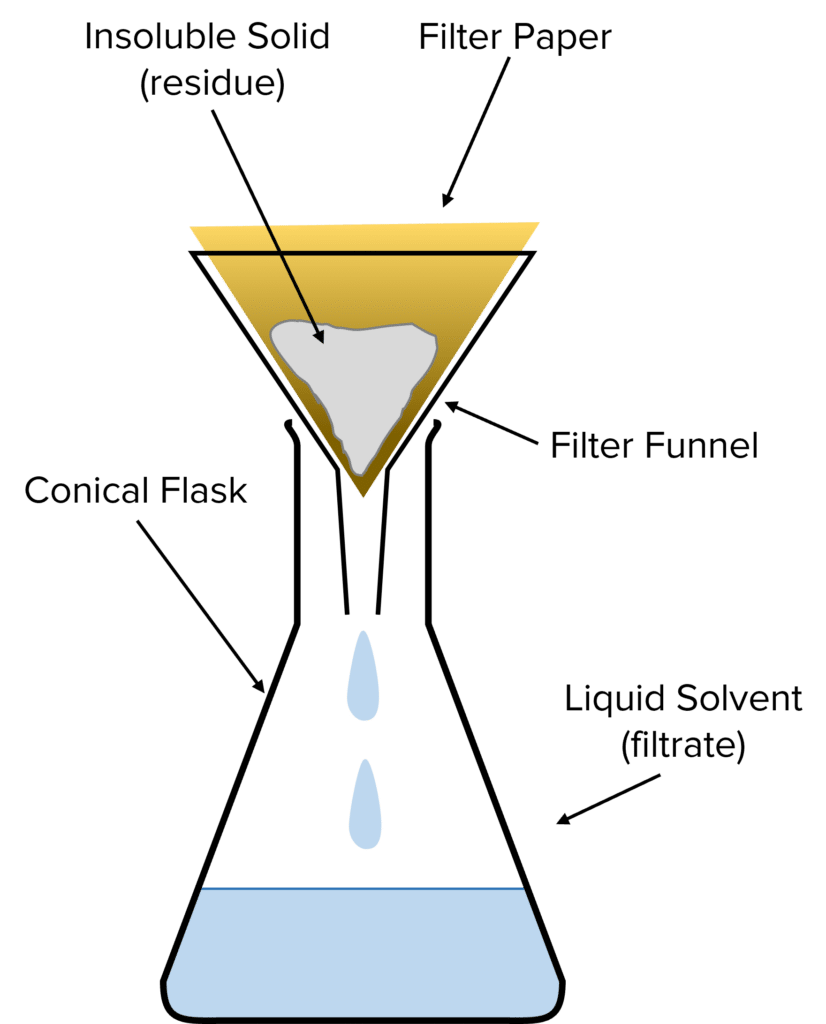
filtration is used to…
separate a solid from a liquid
(two solids if one is soluble in water)
describe a filtration experiment
use a funnel and filter paper in a beaker
substance left in filter paper (sand) = residue
liquid that comes through (water) = filtrate
crystallisation can be used to…
separate a solute (one which dissolved) from a solution
how to make pure salt from rock salt
crush the rock salt + mix with hot water, salt dissolves and the impurities dont
filter off the impurities, leave them on filter paper
filtrate is called salt solution
you can get the solid salt from the solution by crystallisation
simple distillation can be used to…
seperate the components of a solution
e.g. water can be separated from salt solution
fractional distillation can be used to…
separate a mixture of liquids with different boiling points
e.g. ethanol and water
Rf value equation
Rf = distance moved by spot/ distant moved by solvent
how a chromatogram provides information about the composition of a mixture
a pure substance produces one spot on the chromatogram
an impure substance, or mixture, produces two or more spots.