Physics topic 3-Particle model of matterr
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
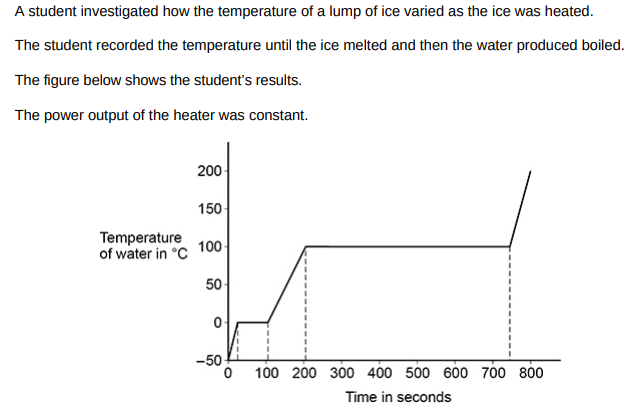
The specific heat capacity of ice is less than the specific heat capacity of water. Explain how the figure above shows this.(2)
The gradient for the ice is steeper than the gradient for the water which means that less energy is needed to increase the temperature by a fixed amound.
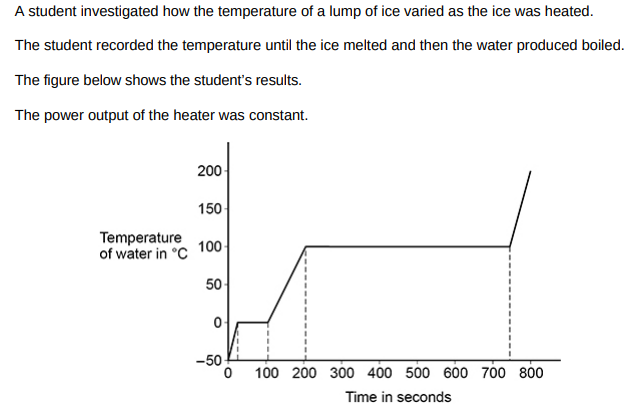
The specific latent heat of fusion of ice is less than the specific latent heat of vaporisation of water. Explain how the figure above shows this.(2)
Water took more time to vaporise than the ice took to melt which means less energy is needed to increase the temperature by a fixed amount.
A second student did the same investigation and recorded the temperature until the water produced boiled. In the second student’s investigation more thermal energy was transferred to the surroundings. Describe two ways the results of the experiment in the figure above would have been different.(2)
Ice/water would take more time to increase in temperature and change state
When the water was boiling, 0.030 kg of water turned into steam. The energy transferred to the water was 69 kJ. Calculate the specific latent heat of vaporisation of water. Give the unit.(5)
69Kj = 69000J, E/m = L, 69000/0.03 = 2300000J/kg
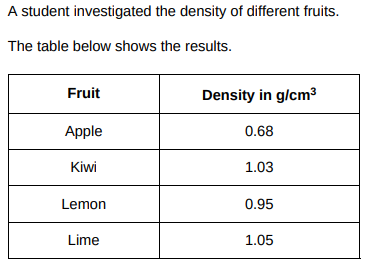
The student determined the volume of each fruit using a displacement can and a measuring cylinder. What other piece of equipment would the student need to determine the density of each fruit?(1)
Scales
Write down the equation which links density (ρ), mass (m) and volume (V).(1)
density=mass/volume
The mass of the apple was 85 g. The density of the apple was 0.68 g/cm3 . Calculate the volume of the apple. Give your answer in cm3 . (3)
V = m/P, V = 85/0.68 = 125
The student only measured the volume of each fruit once. The volume measurements cannot be used to show that the method to measure volume gives precise readings. Give the reason why(1)
repeat readings need taking to show that the readings are close together
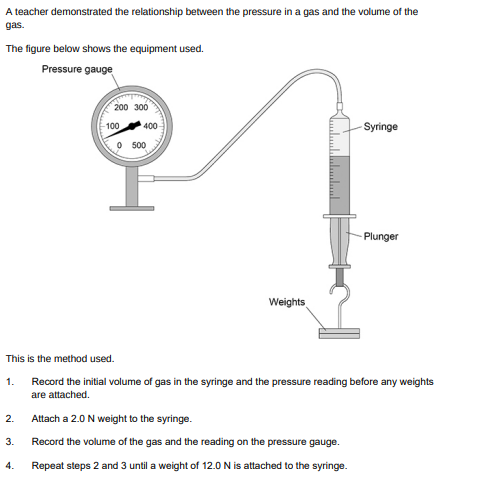
What was the range of force used?(1)
0 to 12
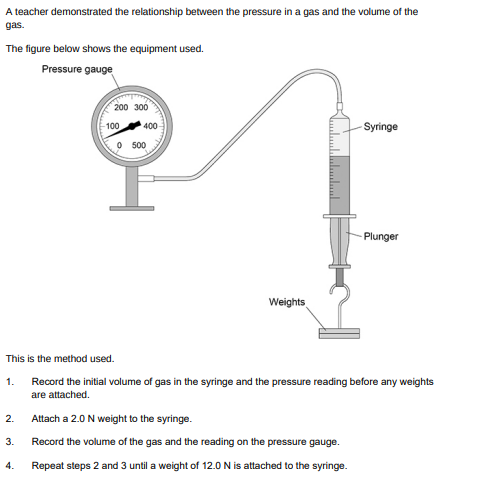
Give one control variable in the investigation (1)
Temperature of the gas
When the volume of gas in the syringe was 45 cm3 , the pressure gauge showed a value of 60 kPa. Calculate the pressure in the gas when the volume of gas in the syringe was 40 cm3 .(4)
Constant = 60×45 = 2700, 2700 = p*40, 2700/40 = p, p = 67.5
When the volume of gas in the syringe increased, the pressure on the inside walls of the syringe decreased. Explain why.(3)
There are less frequent collisions between the particles and the walls of the syringe causing a lower average force on the walls of the syringe and pressure is the total force unit area.

Which statements describe the movement of the gas particles in the balloon?(2)
The particles move in random directions, the particles move with a range of speeds.
The pressure of the helium in the balloon is 100 000 Pa. The volume of the balloon is 0.030 m3 . The balloon is compressed at a constant temperature causing the volume to decrease to 0.025 m3 . No helium leaves the balloon. Calculate the new pressure in the balloon. (4)
constant = 100000×0.030, constant = 3000, 3000 = 0.025*p, p = 3000/0.025, p = 120000Pa
The temperature of the helium in the balloon was increased. The mass and volume of helium in the balloon remained constant. Explain why the pressure exerted by the helium inside the balloon would increase.(4)
Particles would have a higher kinetic energy so there are increased number of collisions with walls of the balloon per second therefore there are greater forces exerted in collisions and on the same area.
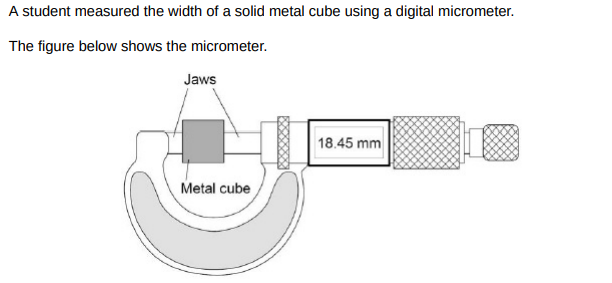
The resolution of the micrometer is 0.01 mm The student could have used a metre rule to measure the width of the cube. Explain how using a metre rule would have affected the accuracy of the student’s measurement of width.(2)
Metre ruler has a lower resolution so it less accurate
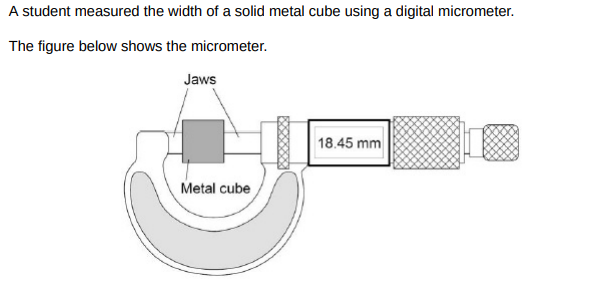
The mass of the metal cube was measured using a top pan balance. The balance had a zero error. Explain how the zero error may be corrected after readings had been taken from the balance.(2)
Record the value of the zero error when there is no object on the balance and subtract the value of the zero error.
The width of the cube was 18.45 mm. The density of the cube was 8.0 × 103 kg/m3 Calculate the mass of the cube.(5)
18.54mm = 0.01845m, V = 0.01845×10**3, V = 6.28×10**-6, 8×10**3 = m/6.28×10**-6, m = 8×10**3×6.28×10**-6, m = 0.0502kg
Explain how the internal energy of the water changes as it is heated from 20 °C to 25 °C.(2)
The kinetic energy of the particles which increases the energy of the water.

How is the particle model used to explain the difference in density between a liquid and a gas?(1)
Particles in a gas have more potential energy than particles in a liquid.
A student measured the mass of boiling water that was turned into steam in five minutes. Explain how the student could use this information to estimate the power output of the Bunsen burner in watts.(4)
Convert 5 mins to 300 seconds.
Energy given to water E = mL with quantities defined
power output = energy transferred/time
power output = change in mass*specific latent heat/time
The student only recorded one set of results. Give two reasons why taking repeat readings could provide more accurate data.(2)
Student can calculate a mean and the student reduces the chance of random error
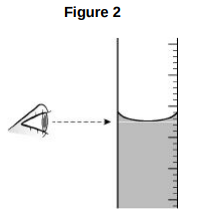
Explain what type of error would be caused if the student’s eye was not in line with the level of the liquid in the tube.(2)
Random error because eye position would not be the same each time
If the gas is compressed too quickly the temperature of the gas increases. Explain how the temperature increase would affect the pressure exerted by the gas. (2)
Temperature would increase pressure in tube as there is higher kinetic energy in particles.
One of the student’s results is given below.
pressure = 1.6 × 10**5 Pa
volume = 9.0 cm3
Calculate the volume of the gas when the pressure was 1.8 × 10**5 Pa. The temperature of the gas was constant.(3)
constant = 1.6×10**5×9, constant = 1.44×10**6, 1.44×10**6 = 1.8×10**5*V, V = 1.44×10**6/1.8×10**5, V = 8cm**3

The internal energy of the air increases as the tyre is inflated. Explain why. (2)
Work is done on the air so its temperature icreases