chem 351 final flashcards (midterm onwards)
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
mass spectrometry - how does it work
1. a sample is injected
2. heater used to make sample into a gas so molecules can diffuse
3. an electron beam ionizes the sample (takes away an electron, making +1 radical cations)
4. particles accelerated in a magnetic field which has a circular path
5. bigger radius means bigger mass, so we can see mass.
6. framgnetation occurs because the molecules break apart in diff places to be more stable
what can we learn from a mass spectra
1. the peak with the highest mass that isnt isotope stuff is M+, the molecular ion - it is the ion formed from the whole molecule
2. if the relationship between M+ and M+2 exists: a 1:1 ratio shows Br is present, a 3:1 ratio shows Cl is present
3. if the molecular weight is odd, there has to be one nitrogen present
NMR - how does it work
atomic nuclei have the property of spin, which means they are magnetically active. # of spin states = 2(nuclear spin quantum number) + 1
spin can be up or down.
if placed in a magnetic field they will align themselves with the field.
diff energies at diff magnetic fields mean diff energy gaps to spin flip.
photon can get absorbed by a molecule if the photon energy equals the exact amount of energy to cause a spin flip.
change in energy is proportional to strength of magnetic field.
what is the J constant for an alkane
around 7 Hz
what is the J constant for a cis alkene
around 10-12 Hz (anywhere from 6-15 Hz)
what is the J constant for a trans alkene
around 12-18 Hz
what is the J constant for a ortho benzene
7-10 Hz
what is the J constant for a meta benzene
2-3 Hz
what is the J constant for a para benzene
0-1 Hz
what is chemical shift
it is a measure of (frequency of signal - frequency of TMS peak) x 10^6/spectrometer frequency
TMS is a reference molecule that we set as 0, tetramethylsilane, because lots of H of the same type.
what is deshielding
electrons have a magnetic field that reduces the magnetic field of the nucleus. the electron density shields the nucleus.
if something is around to change and reduce the electron density around an H or C nucleus, the chemical shift increases and is deshielded.
Inductive effects can deshield by moving electrons away from C or H and towards the electronegative atom. More electronegative or more electronegative atoms or closer electronegative atoms increases deshielding.
hydrogen bonds increase deshielding.
what is magnetic anisotropy
non uniform magnetic field - electrons in pi systems interact with applied fields to create their own fields which causes anisotropy.
around an alkene or a benzene ring, there is a larger field experience by C and H due to double bonds which causes very deshielded.
around an alkyne, H's are out of the range of the magnetic field because applied field and field due to the pi are in opposite directions, so a smaller field is experienced with smaller shifts.
what can you learn from a CNMR
1. the number of types of C
2. what kind of C's they could theoretically be according to the data tables
what does each part of a HNMR tell you
1. the shift
- tells you how deshielded the thing is
- is it close to the substituent, or the farthest away
- look at data sheets
2. how many peaks
-tells you how many types of H
3. integration
- tells you how many of that type of H there are
-the area of a peak is proportional to the number of H absorbing photons at that frequency
4. coupling patterns
-because the magnetic field a proton experiences is influenced by the magnetic field of other neighbouring protons.
-J is the coupling constant that is the strength of the interaction of the protons and the separation of the adj lines.
-tells you how many hydrogen neighbours each hydrogen has
- number of neighbours is lines - 1
coupling issues
OH does not couple
equivelant protons do not couple
accidental equivelance like in benzenes, - all types of benzene H will look like one type of H
what are the two parts of a nucleophilic reaction
Nu - the nucleophile (the thing that attacks the electrophile and donates electrons)
LG - leaving group (the displaced group after nucleophile attacks, or before)
what makes something a good nucleophile
wants to be an electron donor
1. has pi bonds to give away, or lone pairs to give away
2. if the same atom, anion is more nucleophilic than neutral
3. if same row of periodic table, less elecronegative is more nucleophilic
4. in the same group, nucleophilicity increases as atom gets larger (opposite to basicity) because it is more polarisable (bigger electron clouds are easier to distort) so bond formation is more likely.
5. minimal or no resonance increased nucleophilicity
what are some very good, good, fair, weak, and very weak nucleophiles
very good: I-, HS-, RS-
good: Br-, OH-, O-, NC-, N3-
fair: NH3, Cl-, F-, RCO2-
weak: H2O, OH
very week: COOH
overall: S- is a great nucleophile, along with O-
Halide ions like I- and Br- are really good.
N- is pretty good.
If there is resonance, not that good.
what makes a good leaving group
the conjugate base of a strong acid.
what are some very good, good, fair, weak, and very weak leaving groups
the best: TsO-, NH3
very good: I- H2O
good: Br-
Fair: Cl-
poor: F-
very poor: OH-, NH2-, RO-
what two bond changes have to happen in a nucleophilic substitution
a new bond form, C - Nu
a bond breaks, C - LG
there can only be a nucleophilic subsitution around an sp3 hybridized carbon
SN2 reactions
-bond changes are simultaneous
-concerted process (one step reaction)
-rate of reaction is k[Nu][R-LG]
-prefers methyl and primary
-happens with really good nucleophiles
-creates an inversion of stereochemistry at chirality centres, like an umbrella flipping over
-prefers polar aprotic solvents that enhance nucleophilicity of the nucleophile
SN1 reaction
-the leaving group bond breaks first, creating a carbocation intermediate (slow step)
-then the nucleophile attacks (fast step)
-rate of reaction is k[R-LG]
-prefers weak allylic/benzylic bonds, tertiary and secondary
-happens with really good leaving groups (the nucleophile doesnt matter)
-since all alkyl carbocations are sp2 hybridized, they are planar, and there is a loss of stereochemistry and racemic products (50% each enantiomer)
-prefers polar solvents to stabilize the carbocation intermediate
why can't CNu bond form, then CLG bond break?
because a carbon cannot have five full bonds, too much strain
If OH is the nucleophile...
the oxygen is the nucleophile that attacks and the H helps extract the leaving group
if OH is the leaving group...
the whole OH leaves usually as water after protonation by an H
Acetylides
terminal alkynes that are acidic.
A stronger base treats the alkyne so that it becomes its conj base which is a good nucleophile with a C-
Alkylation reaction
SN2
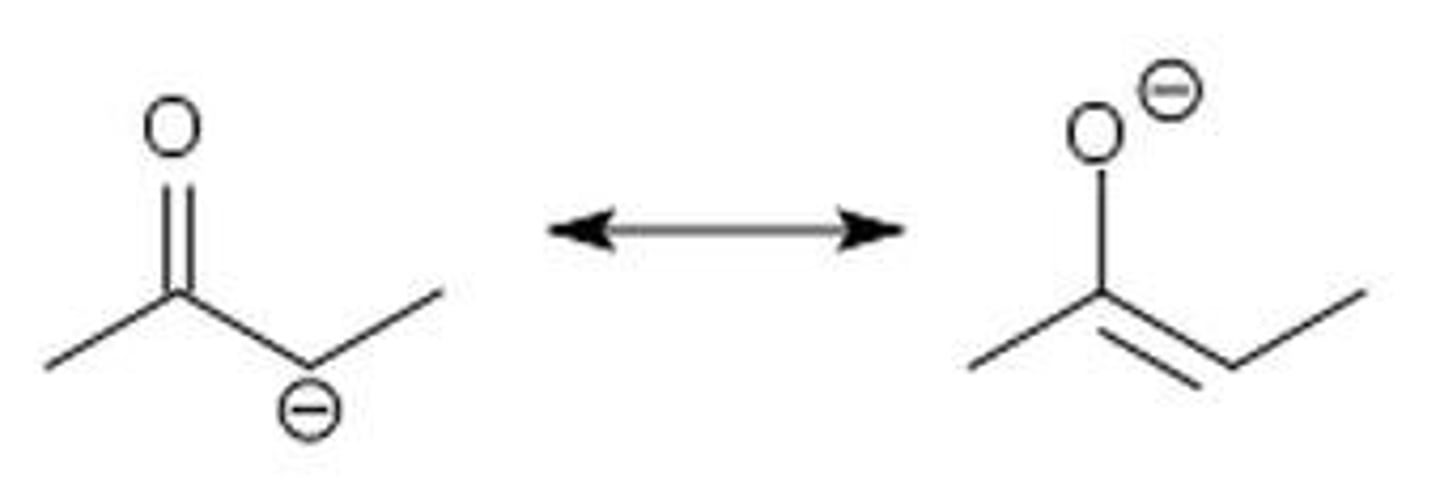
Enolates
when there is a carbonyl (C=O) and the alpha carbon (C-C=O) loses a hydrogen and now has a negative charge
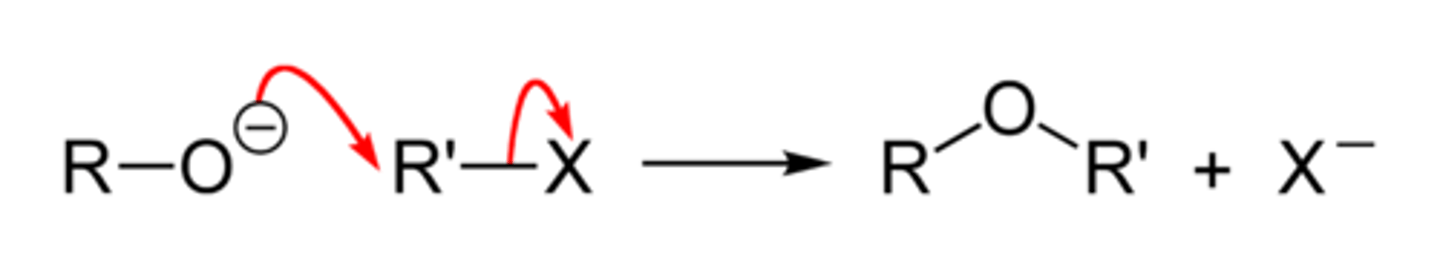
Williamson ether synthesis
Alcohol is reacted with sodium and RX to make a nucleophile ether (sodium metal) and hydrogen gas
Mechanism not needed to be known, but it makes an ether
1. R-OH reacted with Na becomes R-O-Na and H(g)
2. R'X reacts with the R-O-Na with the O- as a nucleophile (negative charged O) to make R-O-R' and NaX
Silyl ether synthesis
Silyl chlorides are more reactive than alklyl chlorides so milder reaction conditions can be used.
Makes an ether.
R'OH reacts with R3Si-Cl and Et3N to make R'-O-SiR3
how do we make OH a better leaving group
Strong acids like HX protonate an OH group to make H3O+ that is a good leaving group (SN1)
Another way to make alkyl halides is using SOCl2 (thionyl chloride) which is Cl2S=O to make TsO
what is the order of HX reactivity for nucleophilic substitution
HI > HBr > HCl
alkyl halide synthesis using thionyl chloride
R-OH in the presence of SOCl2 and either Et3N or pyridine forms R-Cl + SO2 (increases) and H-Et3N and Cl- ions
Leaving group is SO2 after OH is protonated and the O binds to S=O and both Cl is lost
Nucleophile is Cl-
SN2
1. Nucleophile O attacks electrophilic S to form an O-S bond
2. Then acid base rxn with the pyridine or Et3N, so the H of the OH is lost.
3. Then a Cl is lost (leaving group)
4. The Cl- that was lost becomes the nucleophile that attacks the C which makes the O-S(Cl)=O group leave, and it becomes replaced with the Cl
5. The Cl attached to the O-S(Cl)=O group breaks off on its own
Using PCL3 or PBr3
creates an SN2 reaction.
In the presence of Et3N or pyridine.
Leaving group is OH after the H is removed by acid base
Nucleophile is Cl3
the conversion of alcohols to tosylates process
Tosylates are a benzene para methyl and a O=S(Cl)=O groups called tosyl chloride
React in prescence of Et3N or pyridine
SN2.
Basically, R-OH in the precense of tosyl chloride and Et3N or pyridine makes R-OTs and H-Cl
1. R-OH forms a bond with the S of the tosyl chloride, with O acting as the nucleophile.
2. an acid base reaction occurs removing the H from the OH
3. The Cl of the tosyl chloride falls off, and becomes a Cl- ion
4. The Cl- ion combines with the H of the OH to make HCl
polar aprotic solvent definition
a polar solvent due to bond dipoles (so probably contain N, O, or other electronegative atom) that is also aprotic, so therefore does not have hydrogen bond. Any electronegative atom has no H attached.
ex. acetone, DMSO, and DMF.
importance of polar aprotic solvents
important for SN2 reactions because they do not dissolve anions very well, unlike polar solvents that do. So the anions which are nucleophiles have enhanced reactivity and nucleophilicity.
what does R-X reacted with X- make in nucleophilic substitution
R-X
what does R-X reacted with H2O/Na2CO3 make in nucleophilic substitution
R-OH
what does R-X reacted with R'OH- make in nucleophilic substitution
ROR'
what does R-X reacted with R'CO2- make in nucleophilic substitution
R-O-C(=O)R'
what does R-X reacted with H2SR' make in nucleophilic substitution
RSR'
what does R-X reacted with R'C(triplebond)C- make in nucleophilic substitution
R-C(triplebond)C-R'
what does R-X reacted with N3- make in nucleophilic substitution
R-N3
what is an elimination reaction
a small molecule is lost during the process.
1,2 elimination or beta-elimination
atoms that are lost come from adjacent C atoms.
what are the two most important methods of elimination reactions
dehydration of alcohols (protonating the alcohol so it becomes H2O, then losing it)
dehydrohalogenation (losing the HX of alkyl halides)
the three steps of an elimination reaction
1. break C-LG bond
2. remove a proton
3. form a C-C pi bond
E1 reactions
the C-LG bond breaks first, then a proton is lost, then a pi bond forms.
Rate = k[R-LG]
Procees through a carbocation intermediate.
Therefore, tertiary and weak allylic bonds are preffered.
Favours more stable alkene as major product.
Good leaving groups.
Prefers low concentrations of weak bases.
Likes polar solvents to stabilize carbocation
E2 reactions
all steps of the elimination happen simultaneously.
Rate = k[B][R-LG].
Concerted process.
Also prefers tertiary and weaker bonds, because substituents want to be far apart and decrease steric interactions.
Prefers high concentrations of strong bases and more reactive bases.
Happens when H-C bond and C-LG bond are co-planar anti, 180 degrees (antiperiplanar is the word for this) or syn (0 degrees)
Poorer leaving groups.
Prefers polar aprotic solvents to enhance the base.
regioselectivity
preferential formation of one constitutional isomer of an alkene over another during an elimination.
Zaitsev's rule
The most substituted alkene will be formed more. This rule is not always obeyed, but when it is, it makes the Zaitsev product.
If rule is not obeyed, you get anti-Zaitsev or Hoffman product.
stereoselectivity
eliminations often favour the more stable trans product over a cis product
the competition between elimination and nucleophilic substitution
heat helps promote an entropy affect.
nucleophilic substitution does not increase entropy, whereas eliminations do by increasing the total number of molecules. therefore, heat prefers elimination.
as base strength increases, elimination reactions are preffered over nucleophilic substitution.
typical reagant for a dehydration of alcohol
very strong acid like H2SO4 or H3PO4 in the precense of heat.
typical reagants for dehydrohalogenation of alkyl halides
strong bases.
NaOCH3/CH3OH
KOH or NaOH/CH3CH2OH
KOt-Bu/t-BuOH or DMSO
carbocation rearrangement
the rearrangement of a carbocation to a more stable carbocation, which causes a change in the hydrocarbon skeleton.
If they CAN rearrange, they will, and any steps/shifts MUST be to an adjacent carbon, and MUST be more stable than previous arrangement.
Ex. 1,2 hydride shift - a hydride moves from C1 to C2
ex. 1,2 alkyl shift - an alkyl (like a methyl) moves from C1 to C2
why do E2 reactions not always favour Zaitsev's rule
because an increase in size and sterics increases the yielf of Hoffman. If you get bigger, it is harder and harder for the base to reach the ideal position. so, instead, it removes the most accessible base which usually makes the least substituded alkene.
the worse the leaving group, the more you will get the Hoffman or anti-Zaitsev.
consequences of E2 stereochemistry and the conformational effect
E2 reactions prefer for the C-H and C-LG bond to be coplanar, 180 degrees (anti) or 0 degrees (syn, which is less common).
In cyclohexane systems, LG has to be axial in order to be anti to a C-H bond. Therefore, a ring flip has to occur on the cyclohexane formation if LG is not already axial (which it probably isnt). E2 is faster than E1, so if there is a t-butyl making a ring lock, it is very difficult to do an E2 reaction.
Reactions are faster if the LG is already axial.
substituents prefer to be what position in rings
theyw ant to be equitorial because axial has higher strain. in face, t-butyl prefers so much that it prevents ring flips.
ring flip
-atoms/groups that were equatorial become axial and vice versa
strain and the three types of strain
a destabilising effect present within molecules due to electrostatic repulsions between electrons in close proximity.
Angle, torsional, and van der waal
angle strain
results when bond angles deviate from their ideal values by being stretched or compressed
torsional strain
repulsion between the electrons in adjacent bonds as they twist into closer and closer proximity, which is why eclipsed formation is so unstable
van der waal strain
due to atoms or groups being too close to each other and repulsing.
steric strain
the sum of all strain present in a molecule
ring strain
strain in a cyclic structure compared to an analogous acyclic structure. due to angle and torsional strain
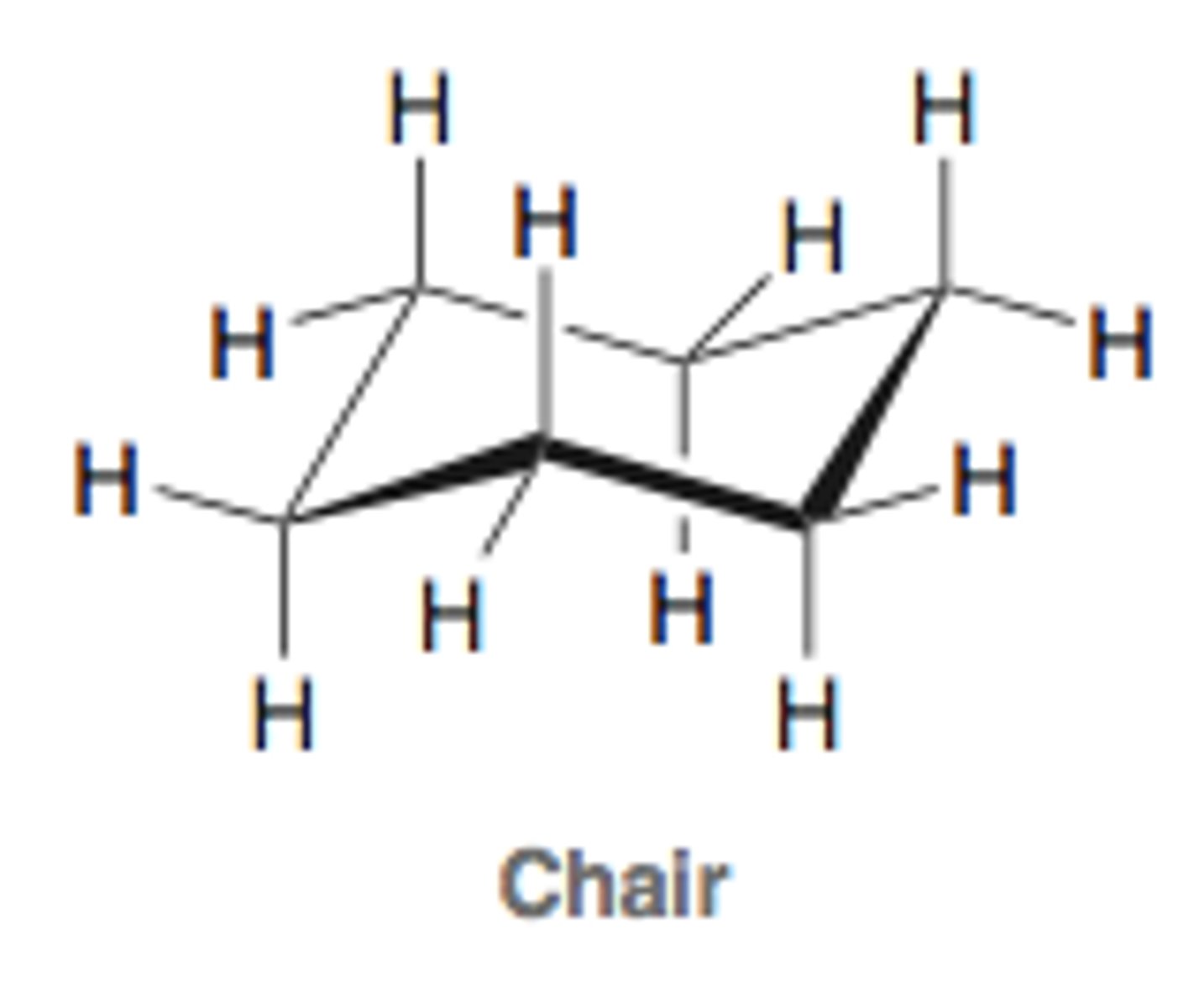
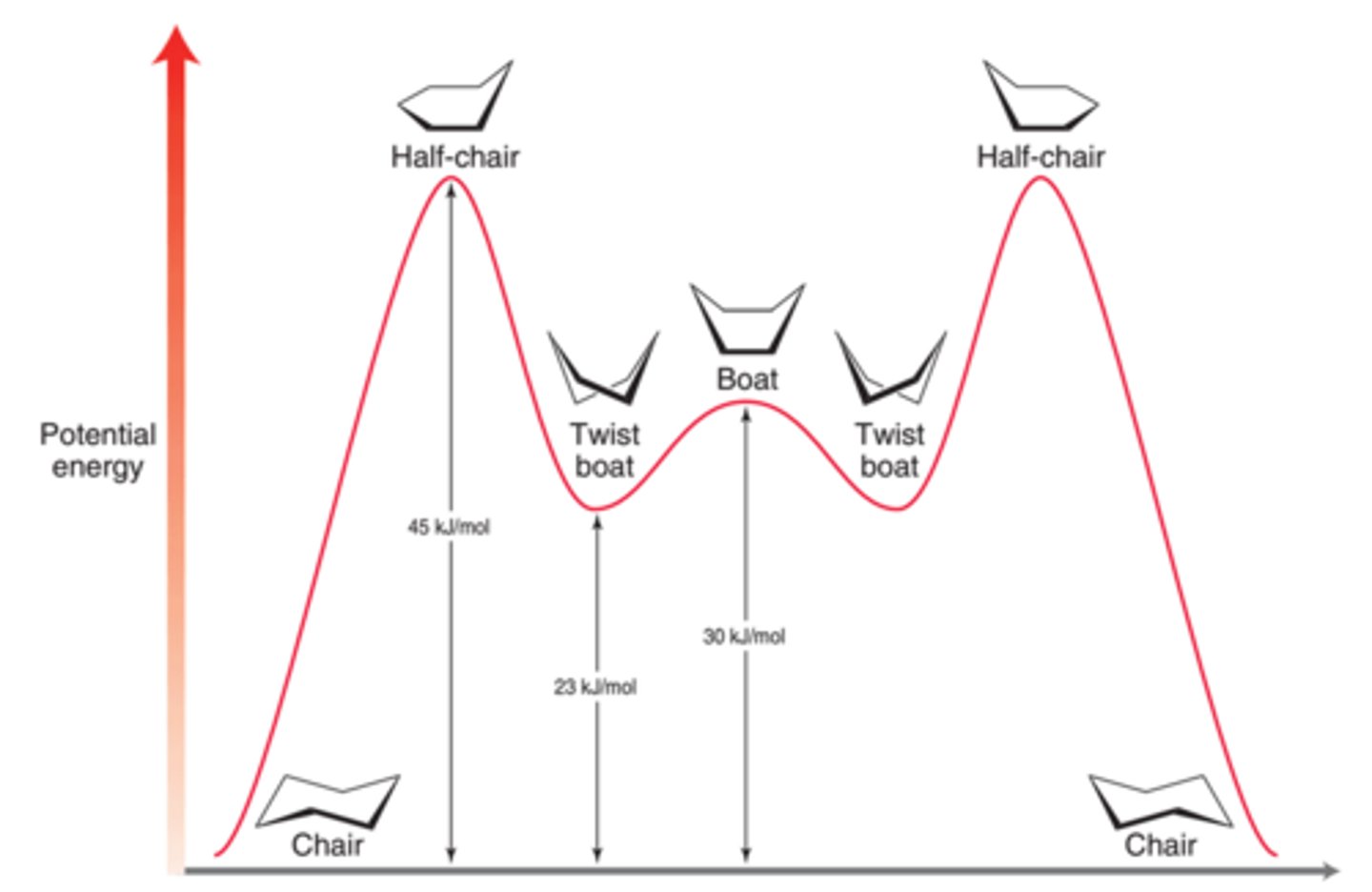
cyclohexane conformations - chair
the most stable conformation.
all bond angles are close to 109.5 degrees, so no angle strain.
all bonds are staggered, so no torsional strain.
it is planar.
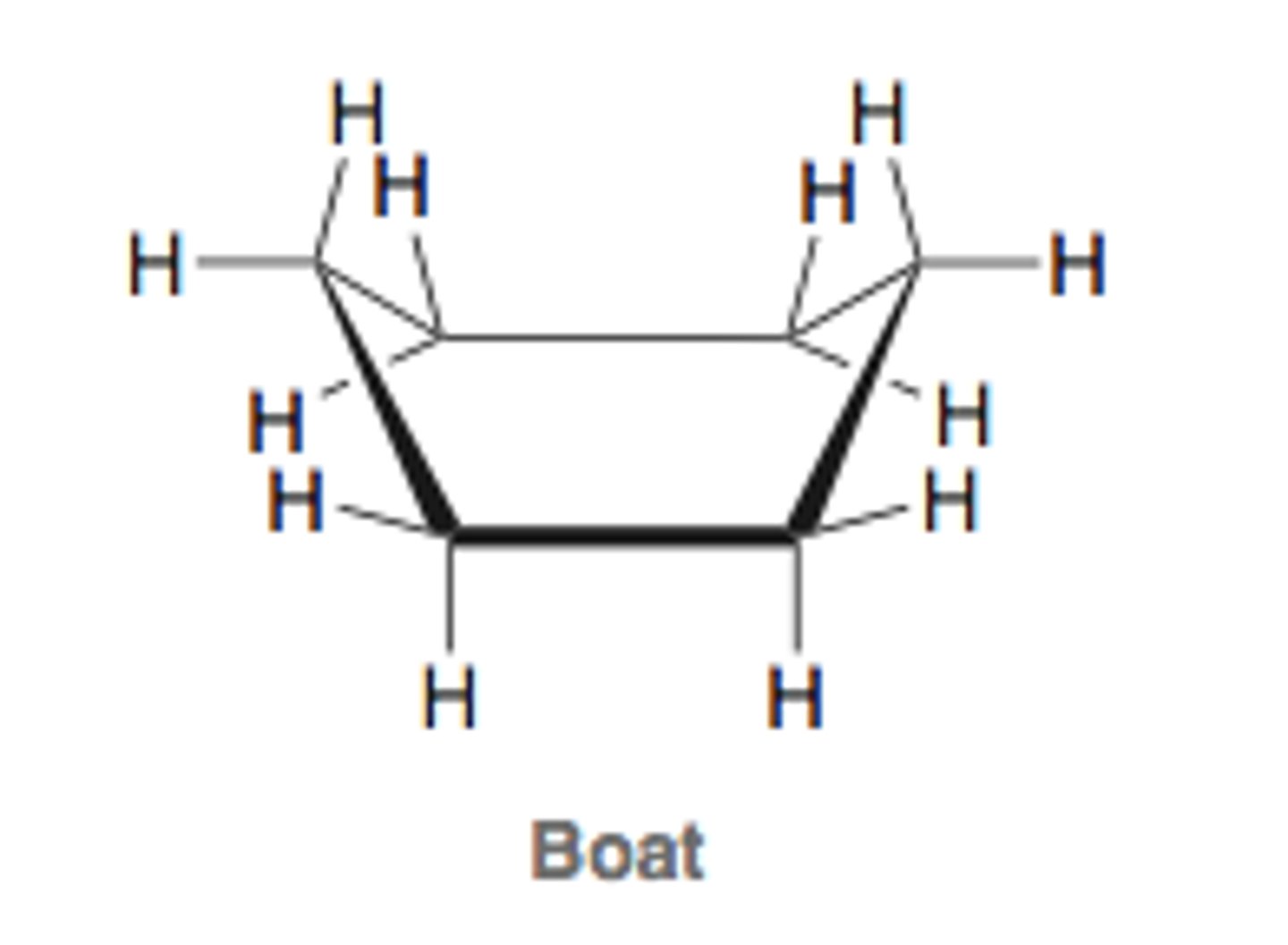
cyclohexane conformations - boat
second most unstable conformation
bond angles are close to 109.5 degrees, so no angle strain.
the conformation is eclipsed along the deck, leading to torsional strain.
the two H attached to the folded over C on each side have a flagpole interaction because they are very close, leading to van der waal strain.
cyclohexane conformations - twist boat
same as boat formation, but there is a little twist so C-H at deck are slightly not eclipsed. moves the H's further apart, so less van der waal and torsional strain.
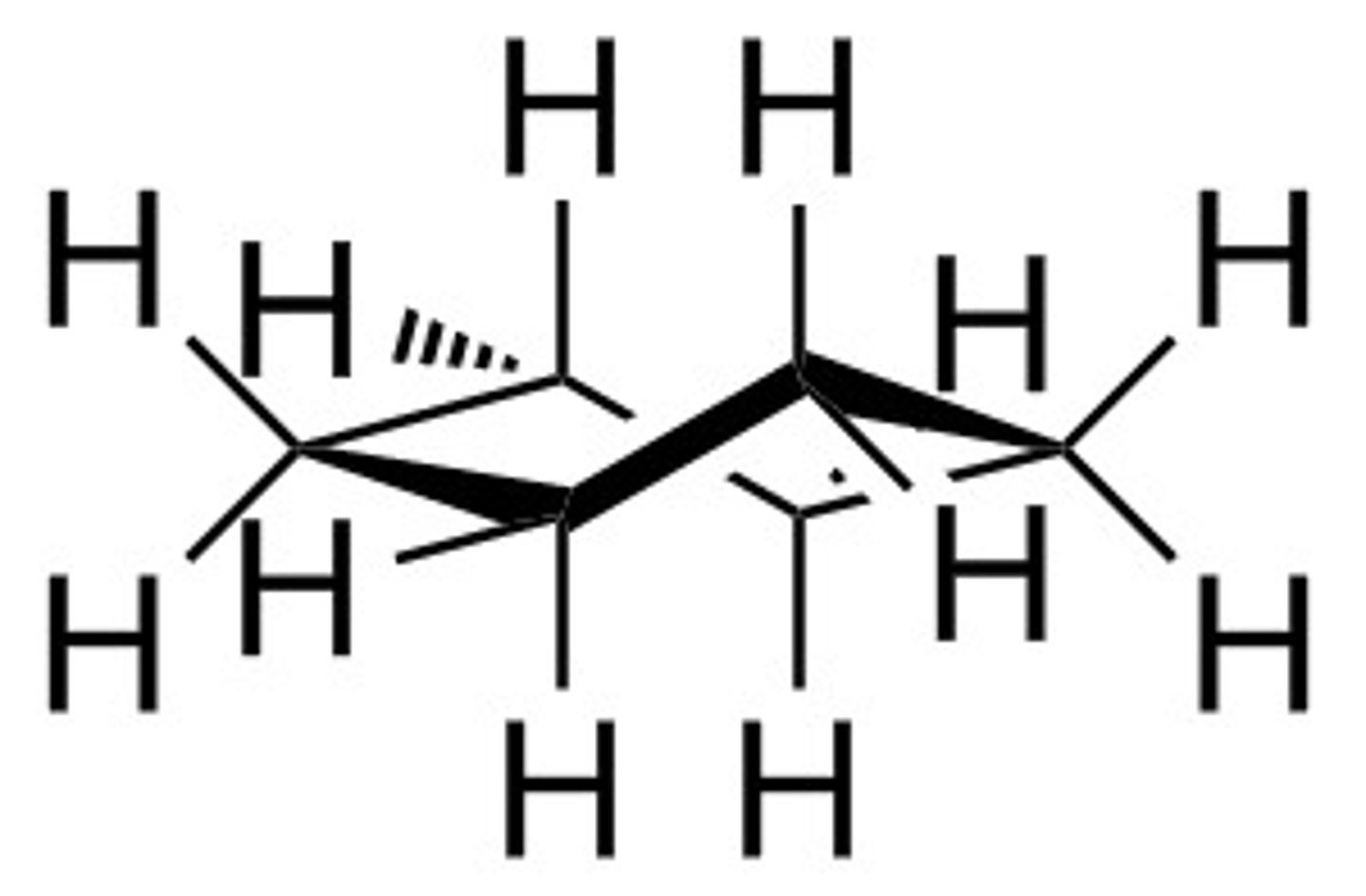
cyclohexane conformations - half chair
some bond angles are distorted, with some eclipsing leading to torsional and angle strain.
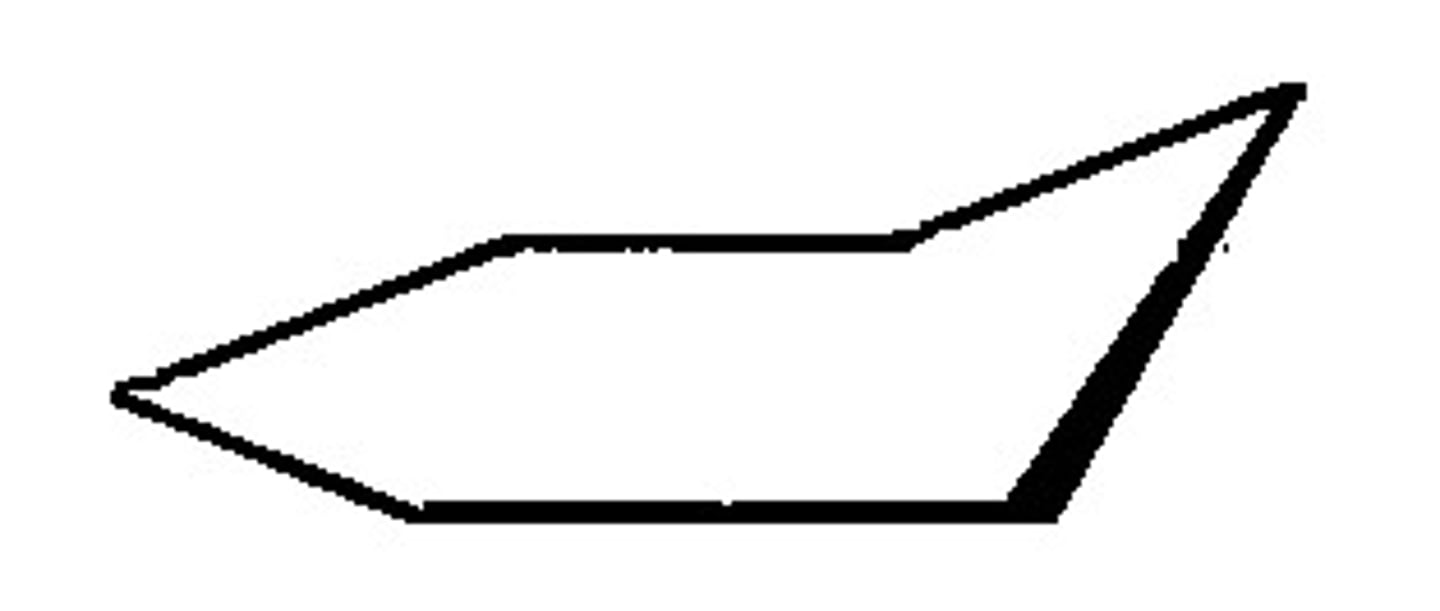
energy diagram of cyclohexane conformations
size and preference for cyclohexane position
as alkyl substituents get larger, there is a greater preference for them to be equitorial.
t-butyl is so big that it prevents ring flips.
if you have two substituents, the cyclohexane will try to be in the position where the larger one is equatorial.