Chemistry - Comparing Metals and Nonmetals
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
the ability to be drawn into wires
ductility
the ability to be hammered or pressed into shapes
malleability
a material's ability to conduct heat
thermal conductivity
a material's ability to conduct electricity
electrical conductivity
the way light interacts with a material's surface
luster
an irregularity within a crystal that occurs at a point on the lattice
point defect
mixtures of two or more elements, at least one of which is a metal
alloy
in metals, including zinc, electrons are not __________ to individual atoms. Instead, they move around ___________, holding them together in a ________________ structure
bound; freely
crystalline
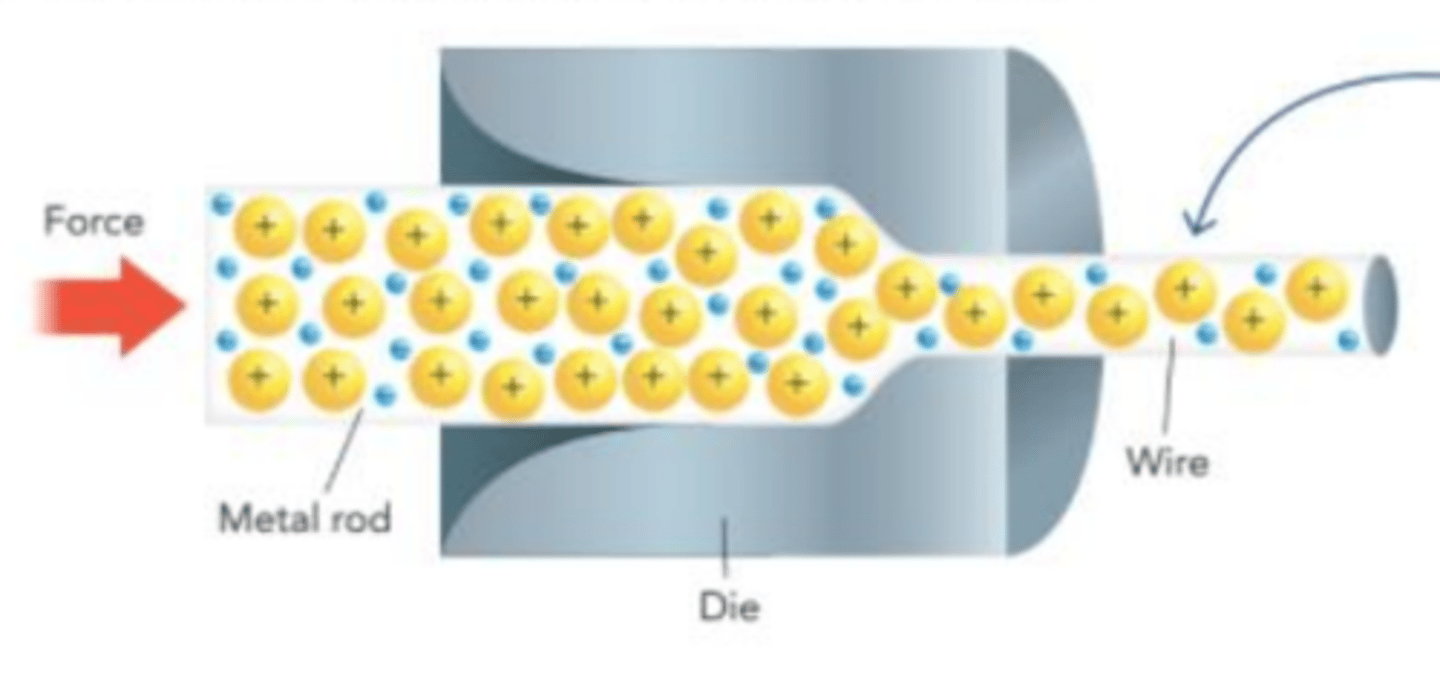
a sea of valence electrons allows metals to be _________________ ________ wire or ______________ without breaking
Ionic compounds __________ behave the same way
squeezed into; hammered
DON'T
Drifting electrons insulate metal cations from each other. When metal is forced through a die, the cations easily slide past one another, allowing the metal to be formed into wire. What is this an example of?
ductility
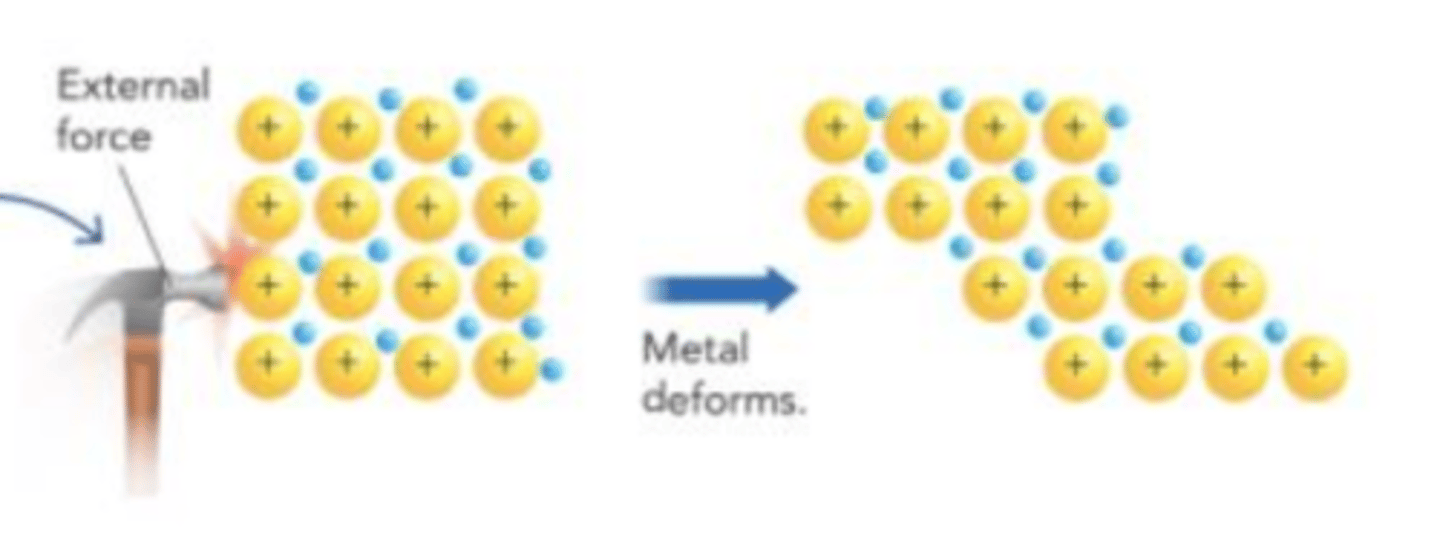
When pressure is applied to a metal, the cations can easily change position, so the metal changes shape without breaking. What is this an example of?
malleability
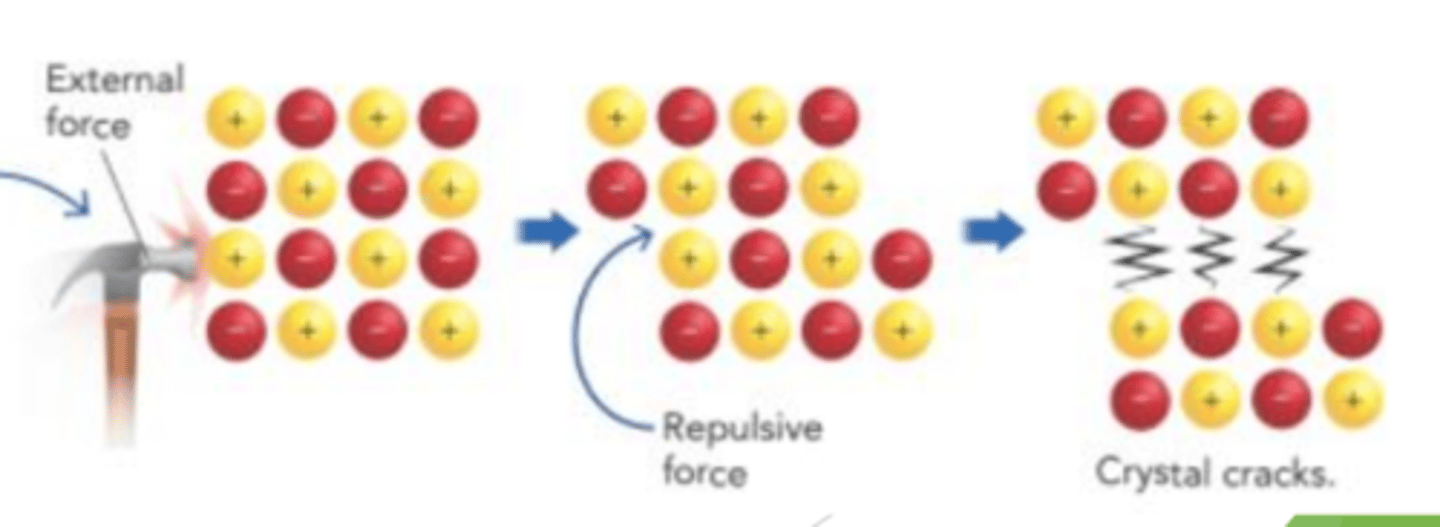
Applying pressure to an ionic compound tends to push the ions close together. The positive ions ________ each other, and the crystal ______________
repel
shatters
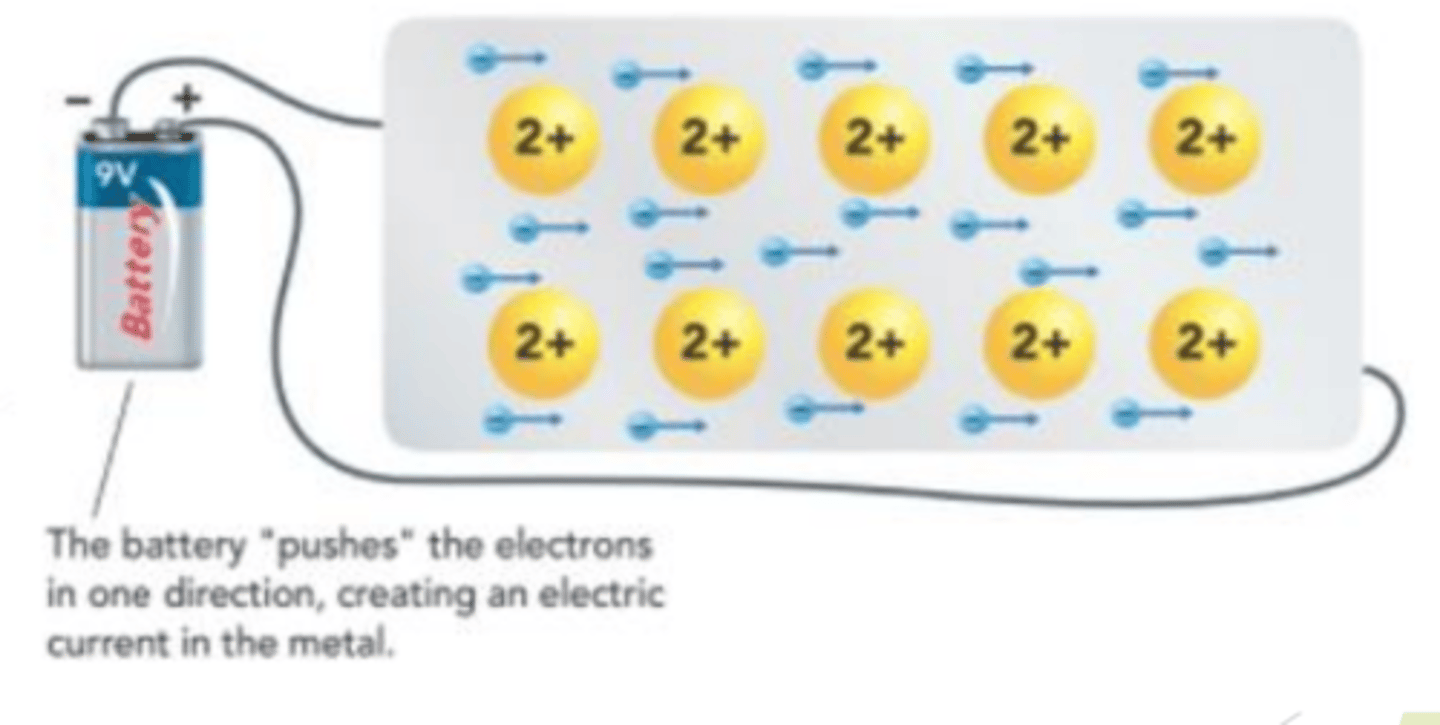
Because electrons can move around, they conduct heat more easily than ______ solids or _____________ ____________ solids.
ionic solids or covalent network solids
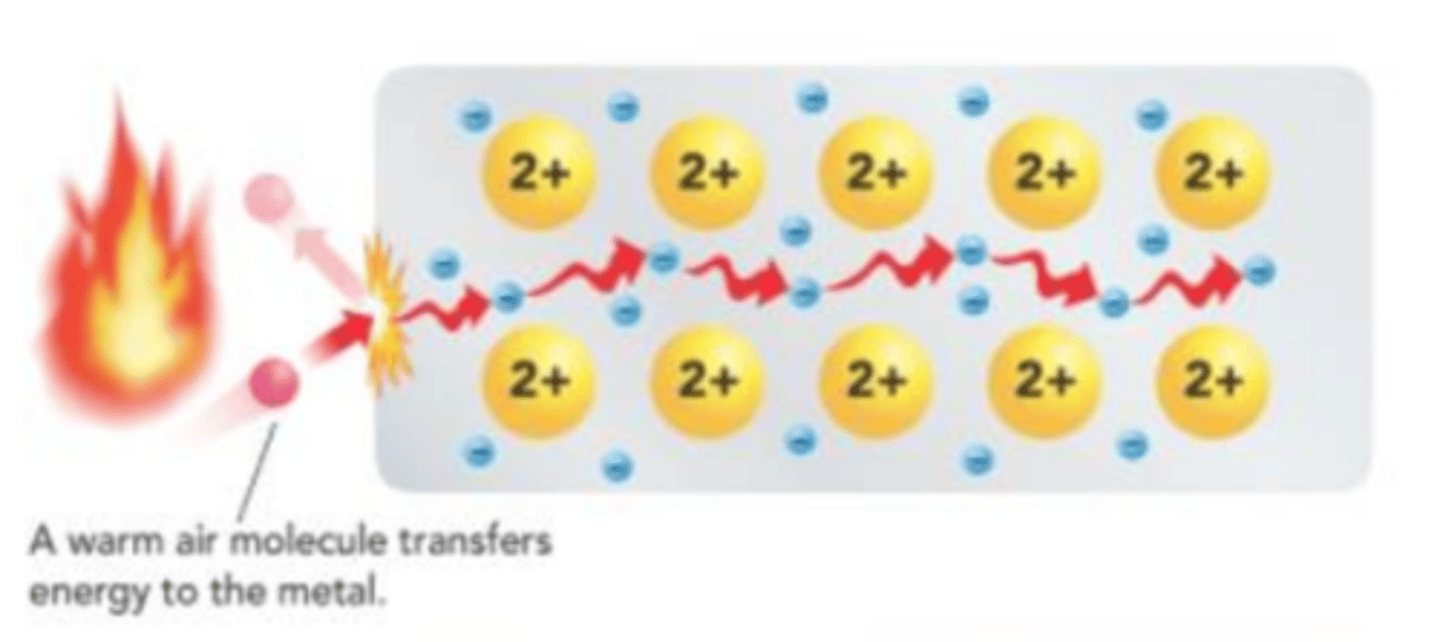
The delocalized electrons in metals can easily move, making metals __________ ________________ than ionic solids
more conductive
In a(n) _______________ defect, extra atoms are stuck where they don't belong
interstitial defect
In a(n) _______________ defect, different atoms take the place of existing atoms in the crystal lattice
substitution defect
In a(n) _______________ defect, atoms are missing from the lattice structure
vacancy defect
the tension on the surface of a water drop
surface tension
a substance that reduces surface tension and increases wetting
surfactant
in any solution, the dissolving medium is called the _______________
solvent
the dissolved particles (usually ions, but also polar molecules) are called the _______________
solute
the process of ion capture by the solvent is called _______________
solvation
a solution where water is the solvent
aqueous solution
a compound that conducts electric current when dissolved in an aqueous solution or in the molten state
electrolyte
a compound that does not conduct electric current in either an aqueous solution or in the molten state
nonelectrolyte
a solid, crystalline compound that contains water molecules as an integral part of its crystal structure
hydrate
the loss of water by a hydrate
efflorescence
a chamber used for maintaining a dry environment in chemistry labs
desiccator
because water is a polar molecule, its molecules are ________________ to each other
_______________ bonds hold water molecules together
attracted
hydrogen bonds
unbalanced forces pulling inward on a drop of water cause it to _________________ the amount of surface area by beading up on some surfaces
minimize
a molecule at the ___________ of the drop will experience only attractive forces from below. Because these attractive forces are ________________ there is a net pull on the molecule into the drop
surface
unbalanced
a molecule in the ____________ of the drop will experience attractive forces from all sides. Because these attractive forces are ______________, there is no net pull on the molecule
middle
balanced
the rate at which a solute dissolves
dissolution rate
contains the maximum amount of solute for a given quantity of solvent at a constant temperature and pressure
saturated solution
the amount of solute that dissolves in a given quantity of a solvent at a specified temperature and pressure to produce a saturated solution
solubility
a graph of the solubility as a function of temperature
solubility curve
areas in the ocean where the oxygen concentration is so low that animal life suffocates
hypoxic zones
contains more solute than it can theoretically hold at a given temperature
supersaturated solution
solutions are called ____________________ _______________ because they have a uniform appearance and composition
homogeneous mixtures
_____________________ _______________ are not uniform in composition and are not considered solutions
heterogeneous mixtures
a heterogeneous mixture containing particles, called the dispersed phase, that are spread throughout another substance, called the dispersion medium
a colloid
a heterogeneous mixture from which particles settle out upon standing
suspension
in a solution, individual molecules and ions of the solute and solvent are _________ ________________
mixed together
most ____________ contain charged particles of clumped molecules or ions that are spread throughout the dispersion medium
colloids
a ________________ is a mixture from which particles settle out upon standing
suspension
when light scatters by particles in a colloid or in a very fine suspension
Tyndall effect
when a beam from a flashlight is directed on beakers known to contain a colloid, a suspension, and solution, which of the following will not exhibit the Tyndall effect?
the solution