Rate constants: Zero and first order kinetics, Half-life, Volume of distribution, Clearance
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Pharmacokinetics
the mathematical analysis of processes of ADME (described best by the rate by which the process proceeds)
What are the 2 orders in pharmacokinetics? Which is most common?
- zero order and first order
- Greater than 95% of drugs follow first order
Rate
refers to the speed a drug progresses through each ADME process
Order
describes how the concentration of the drug influences the rate of ADME processes
Rate constants (K)
specific values that represent the speed of each individual process under defined characteristics
What is the difference between the rate and rate constant of a process?
While rate constant is a constant (does not change with time) and depends on the characteristics of the process, the rate may or may not be constant)
For zero-order kinetics, is the rate of the process constant or changing?
rate is constant at all the time
- is the same as the zero-order rate constant
(DIFFERENT FROM HALF-LIFE, where half-times vary)
In first-order kinetics, is the rate of the process constant or changing?
rate of process would change with time because A would change with time
(DIFFERENT FROM HALF-LIFE, where have times are the same)
If Drug A is degraded to product B, how would the rate of forward reaction be expressed?
-dA/dt
(negative sign shows that the concentration of drug A decreases over time T)
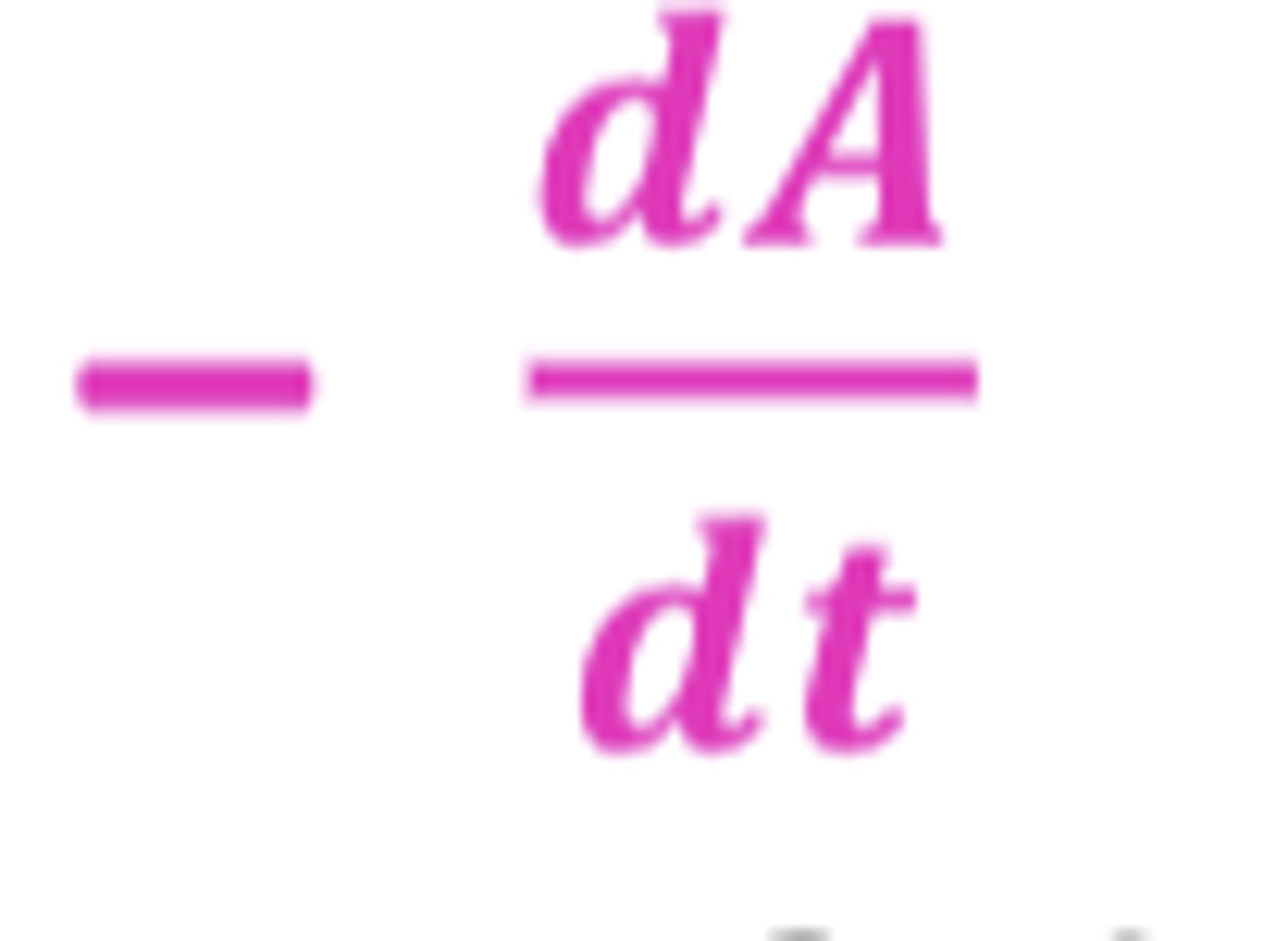
As the reaction continues, the concentration of Drug B increases, how is the rate of the reaction expressed?
dB/dt
(positive sign shows that the concentration of drug B increases over time T)
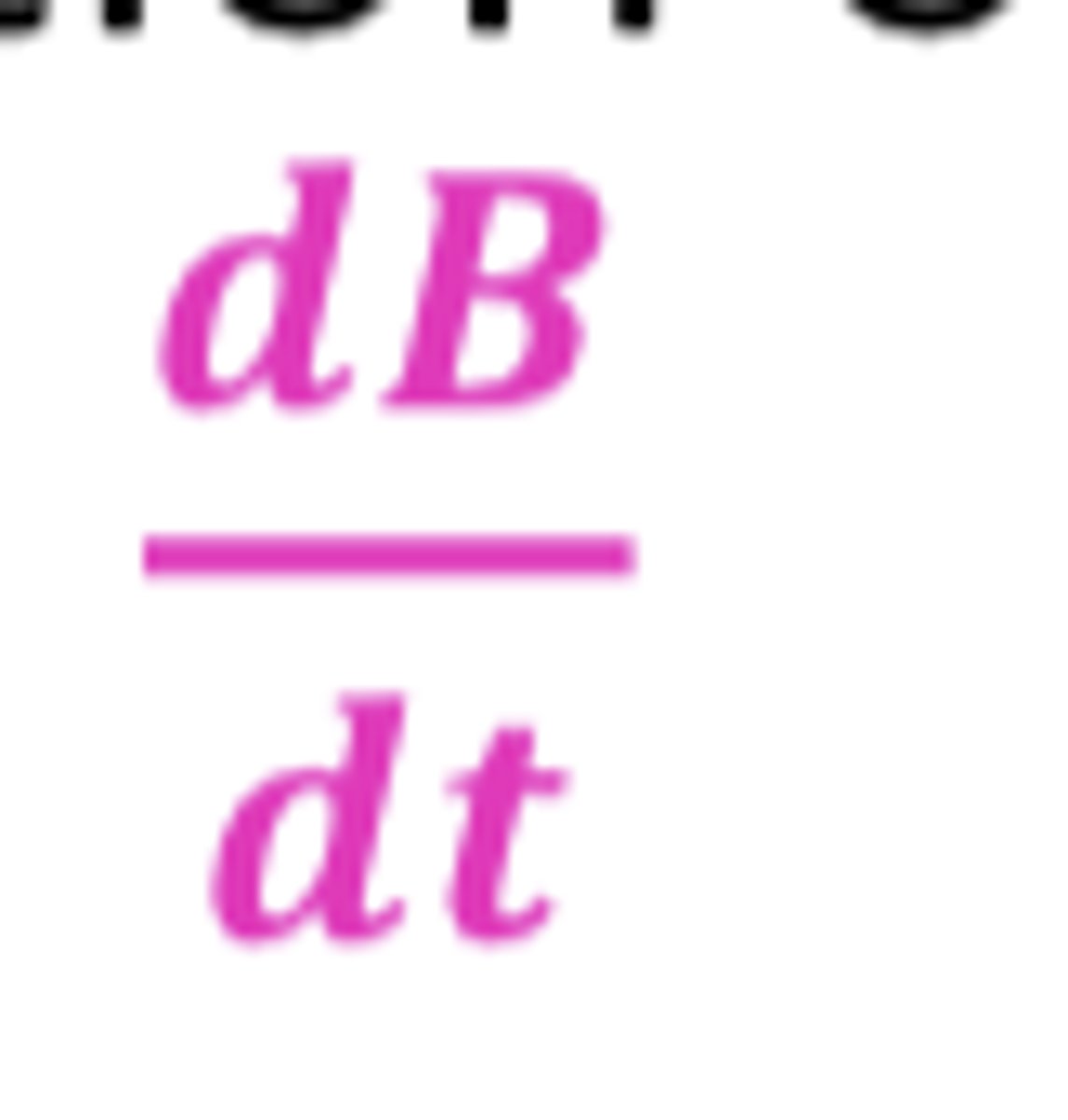
What is K?
the proportionality constant, a rate constant
- units are h^-1
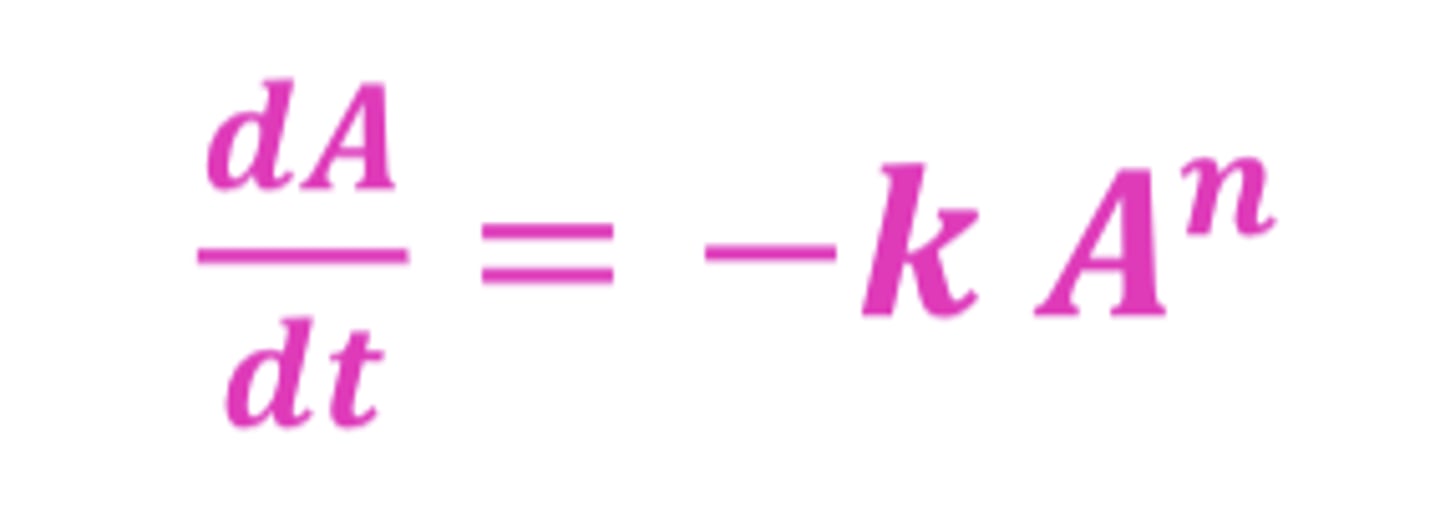
What is n?
order of kinetics process (zero or first)
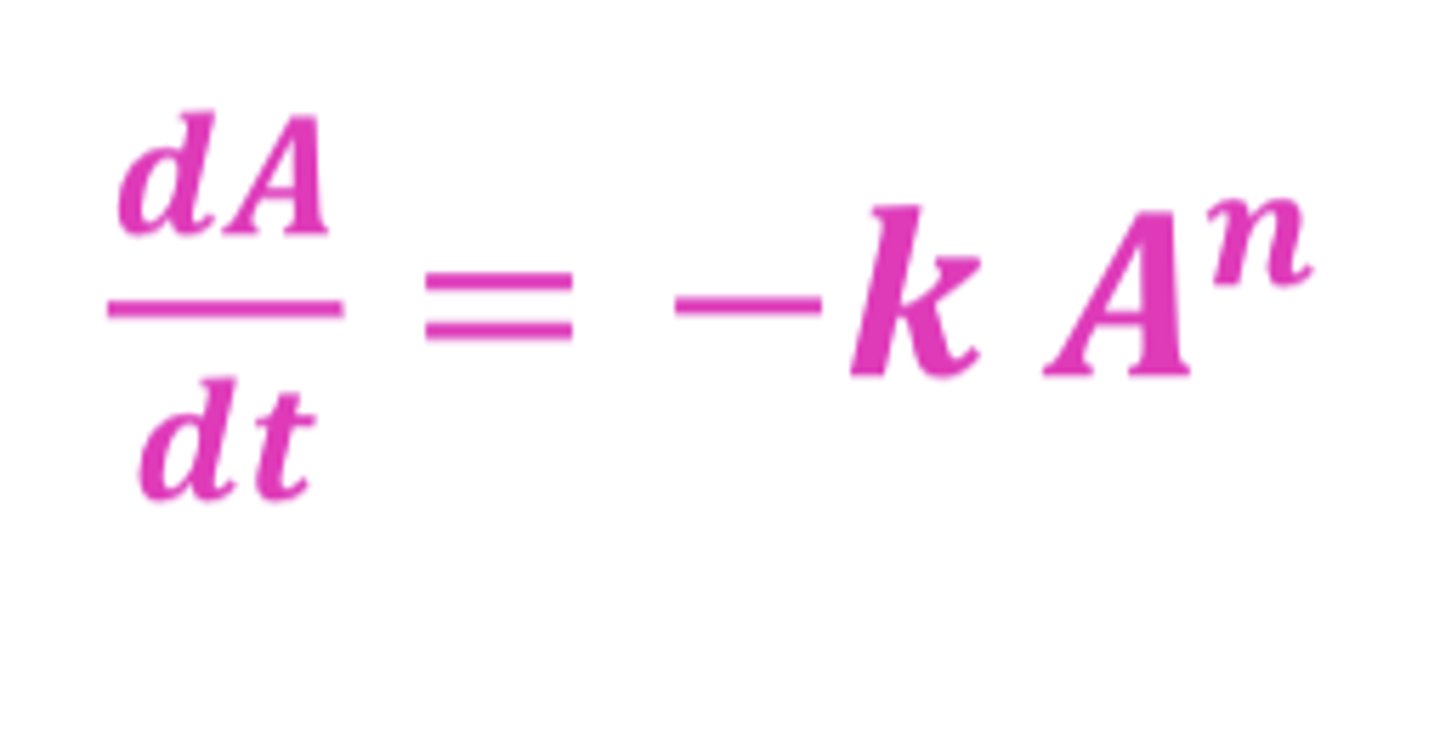
If n=1, what is the order of reaction?
First-order
(2^1=2, A^1=A)
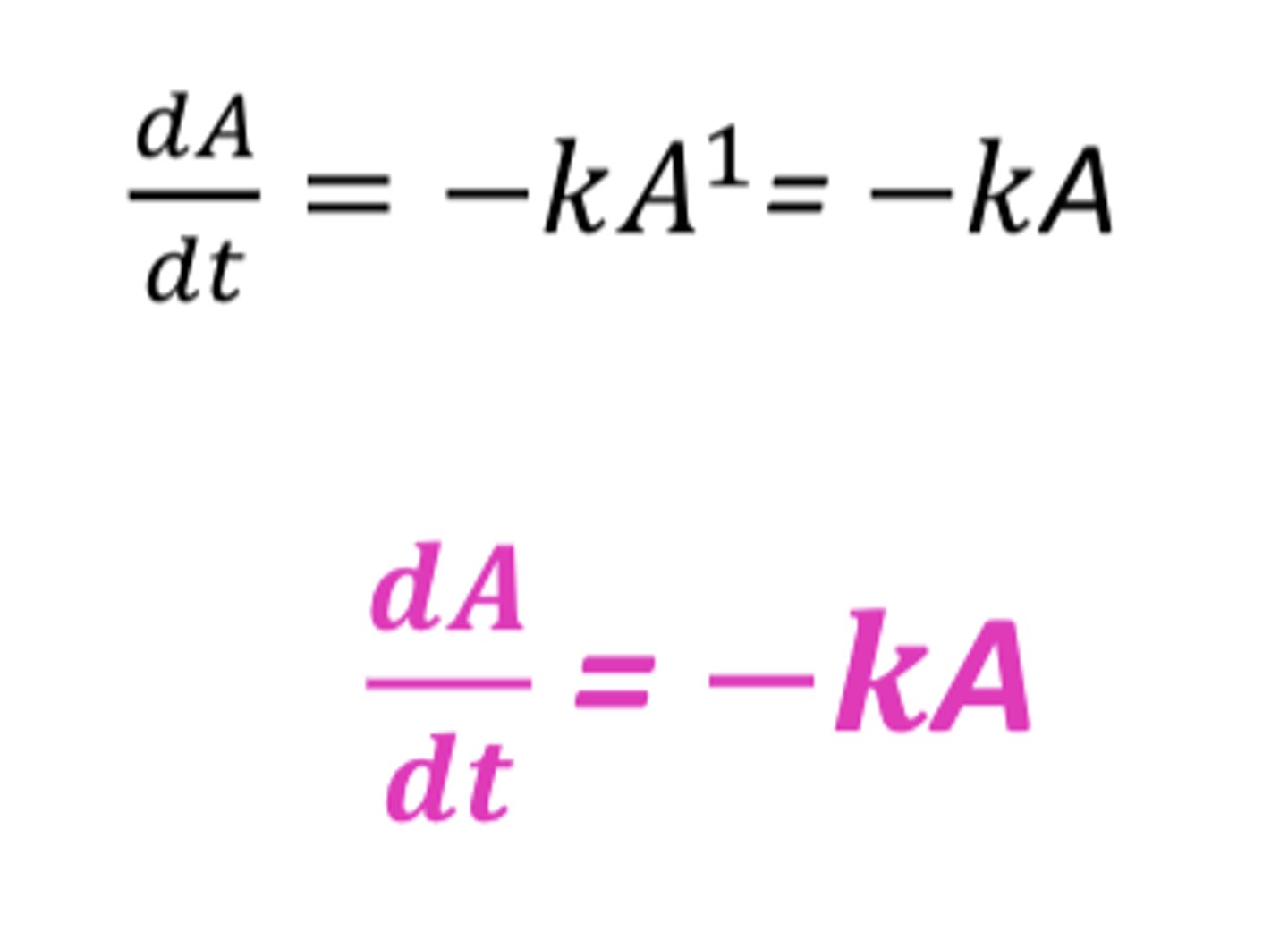
If n=0, what is the order of reaction?
zero-order
(2^0=1, A^0=1)
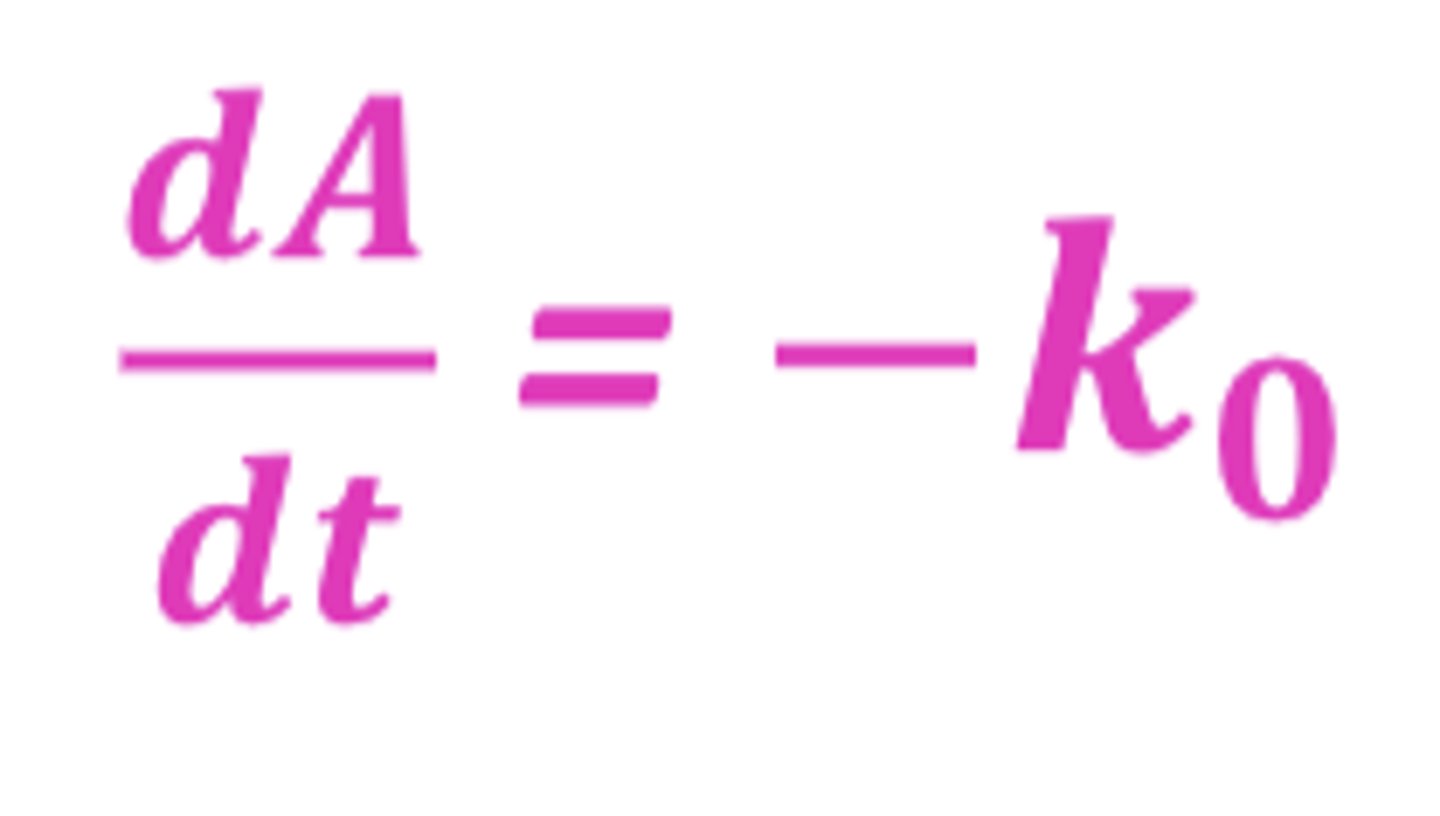
In a first-order process, the rate is directly proportional to....
the concentration of drug undergoing reaction
(the greater the concentration, the faster the reaction)
Why do first-order processes follow linear kinetics?
because of the proportionality between the rate of reaction and the concentration of drug
First Order Kinetics Equation (2)
1. A = A0e^-kt
2. Cp = Cp0e^-kt
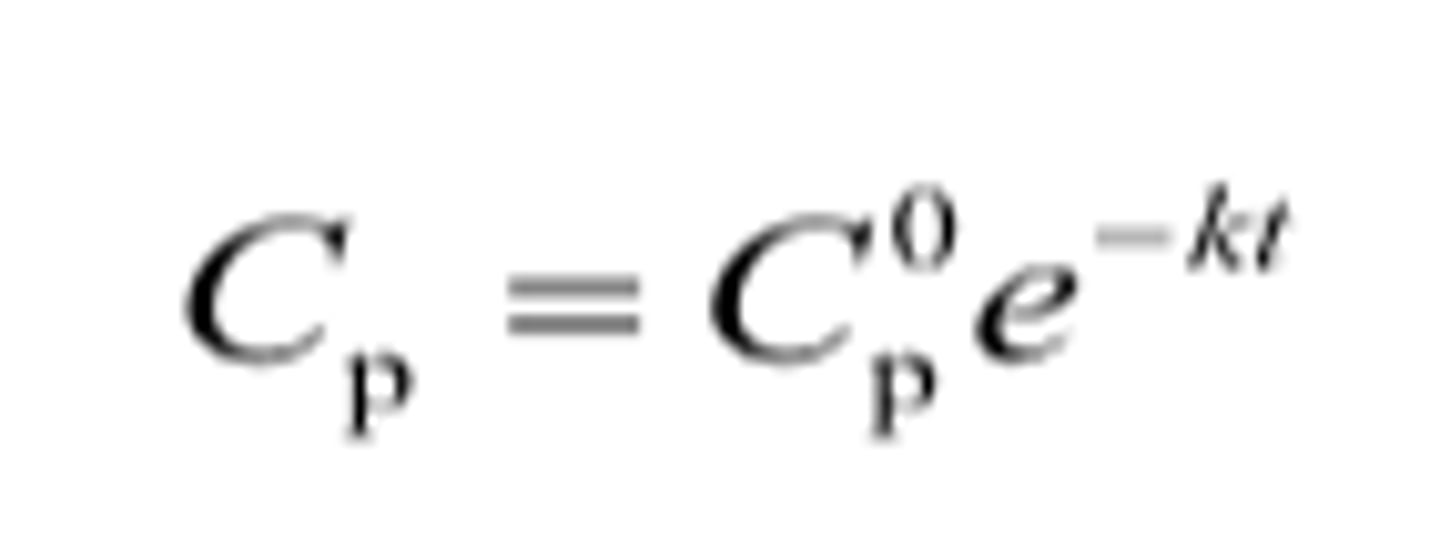
First Order Kinetics Equation:
A = ?
A0 = ?
k = ?
t = ?
A = concentration of drug
A0 = initial dose of drug
k = rate constant
t = time
Half-life (t½)
the time required for the concentration of a drug to decrease to half of its initial concentration
Half-life (t½) formula first-order
t½ = 0.693/k
- units of t½=min,hour,day
- units of k= hour^-1 for 1st order
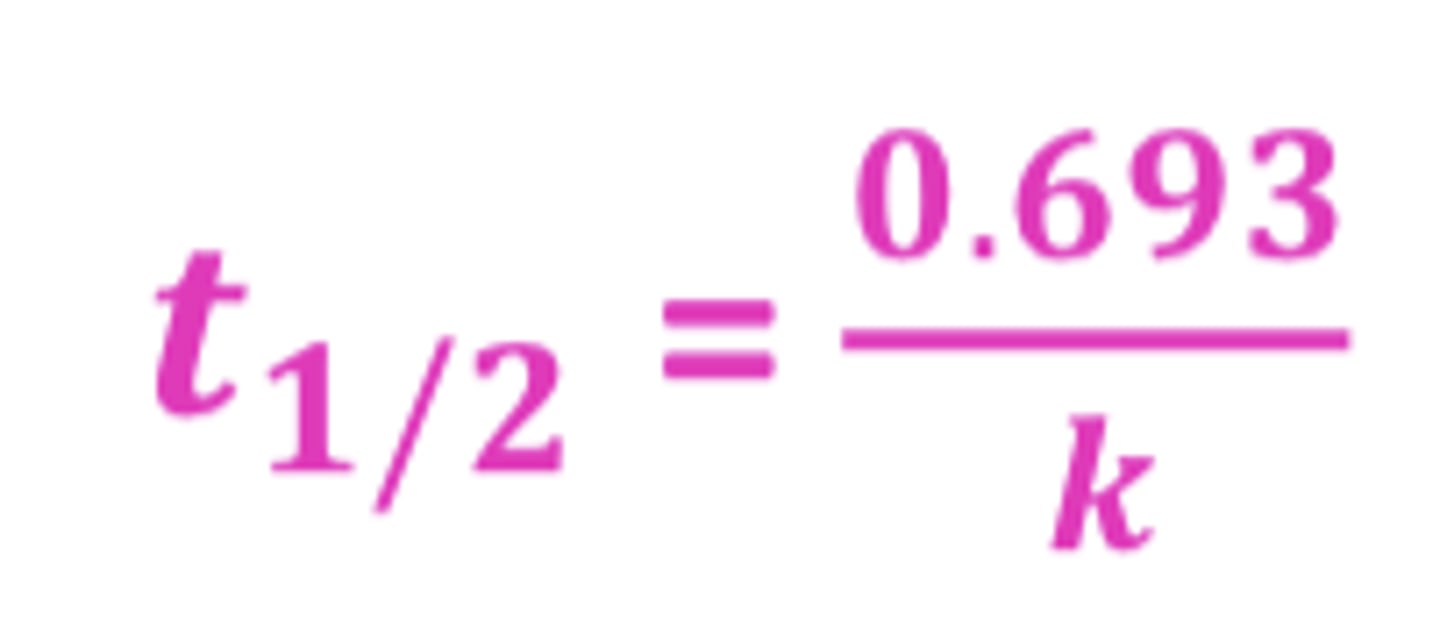
For first-order kinetics, is t½ constant or different?
t½ is constant regardless of the starting concentration [A]
(ex. the the half life is the same, half less each 2 hours)
![<p>t½ is constant regardless of the starting concentration [A]</p><p>(ex. the the half life is the same, half less each 2 hours)</p>](https://knowt-user-attachments.s3.amazonaws.com/7f9b0a5e-a8c4-42ef-a852-b7c6aab46b69.jpg)
T/F: For a first-order process, the half-life DOES depend on [A] (conc. of drug)
FALSE
for first-order the half-life does NOT depend on [A]
In zero-order processes, does the half-life depend on [A]?
Yes, it does depend on [A] (the initial concentration)
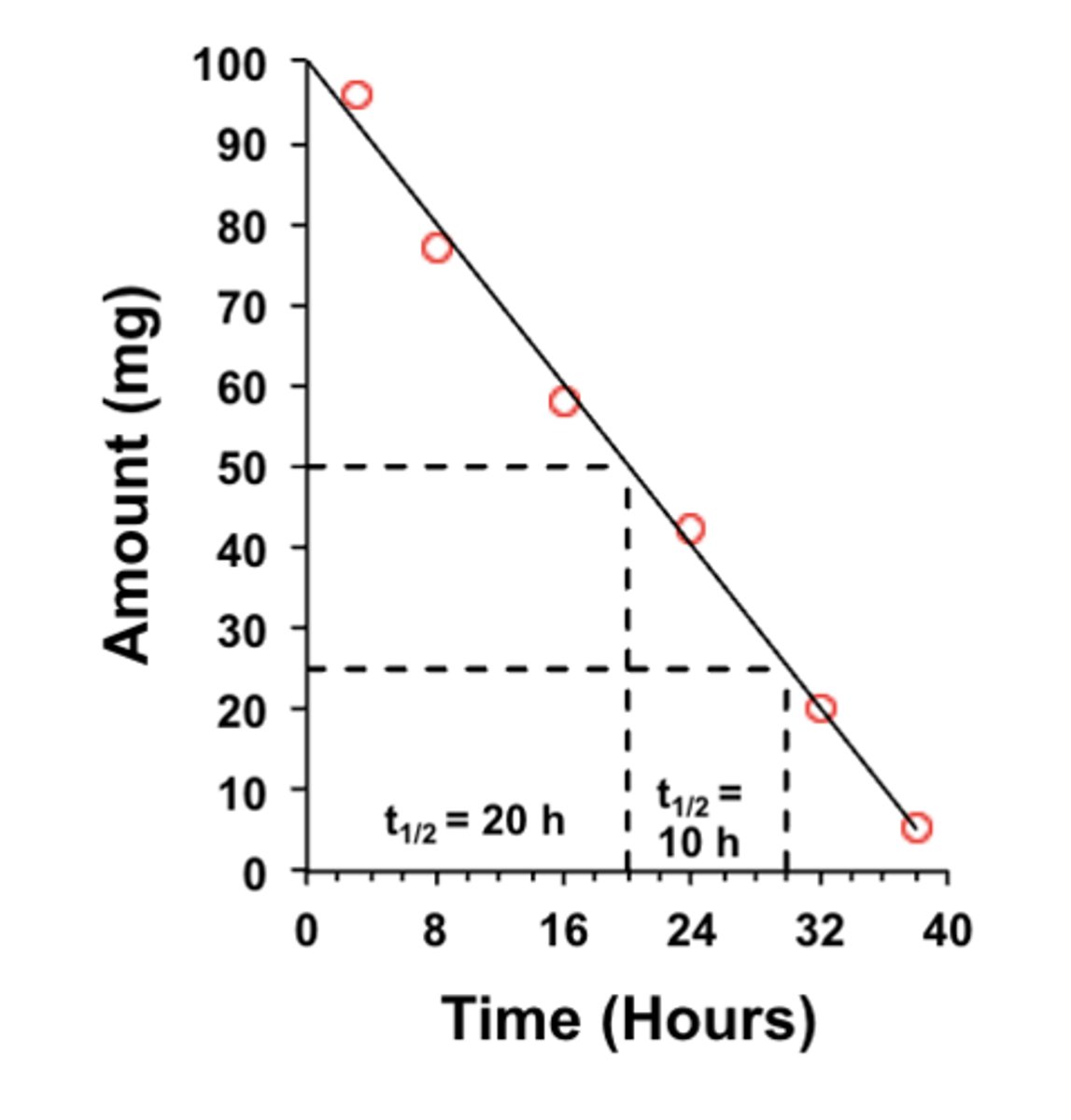
For zero-order kinetics, is t½ constant or different?
it may be different
- it depends on the initial concentration from which decline is measured
(ex: in the graph, t½ is different)
Equation to find K from time and concentration (to be plugged into half-life equation)
K = lnC1 - lnC2/t2-t1
C1 = first concentration
C2 = last concentration
t2 = last time
t1 = first time
Are zero-order processes defined as one whose rate is dependent or independent of the concentration of drug?
INDEPENDENT of the concentration of drug
(rate cannot be increased by increasing the concentration)
Half-life (t½) formula zero-order
t½ = 0.5[A]0/k
![<p>t½ = 0.5[A]0/k</p>](https://knowt-user-attachments.s3.amazonaws.com/45c534ef-dbec-4946-85ea-0fb23d07350c.jpg)
How to find K when only t½ is given
K=0.693/t½
Volume of Distribution (VD)
described the extent to which a drug is distributed in body tissues relative to plasma
"fraction" of drug stays in blood, "fraction" resides in periphery
Volume of Distribution (VD) formula
VD = D0/Cp0
D0 = initial drug amount
Cp0 = initial drug concentration
(in units of volume/weight: ml, L, or ml/kg, L/kg)

Many drugs bind to plasma proteins such as....
albumin, a-1-acid glycoprotein, lipoprotein
An increase in plasma protein binding will....
decrease the volume of distribution, vice versa
Free (unbound) drug is considered an...
active 'species'
- crosses membranes easier
- binds to drug receptors easier
Clearance (Cl)
the volume of blood fluid that is completely cleared from the drug per unit time
- efficiency of eliminating organs
- Units: volume/time (L/hr, mL/min), volume/time/weight (L/hr/kg)
Clearance (Cl) formula
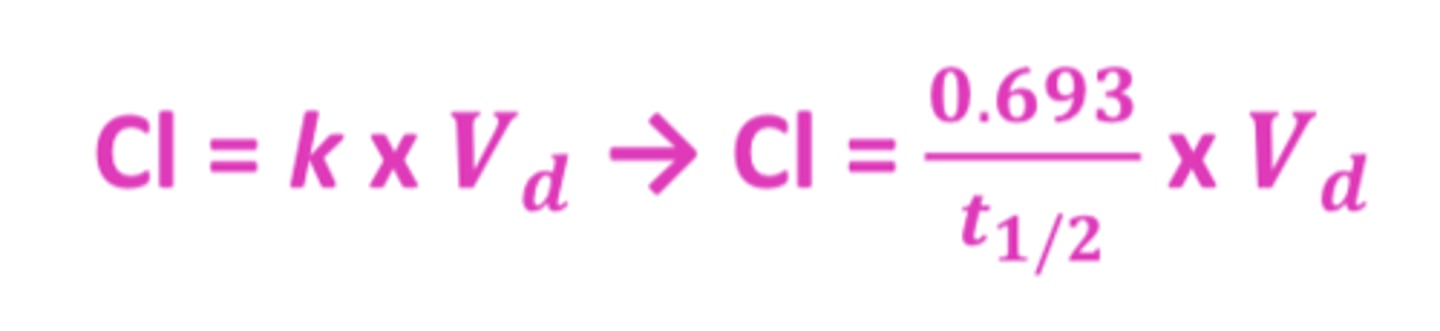
If a decrease in Cl occurs while Vd remains unchanged.... (3)
- slower overall rate of elimination
- concern of drug accumulation and toxicities
- may require dosage adjustments (ex. pts with hepatic and renal insufficiency, increase dosing interval)
If an increase in Cl occurs while Vd remains unchanged... (2)
- faster overall rate of elimination
- may require dose/dose interval adjustments (increase dose or decrease dosing interval)