MLT 110 Exam 2
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
A noncritical measurement can be:
made using a volumetric flask
made using a volumetric pipet
made using an Erlenmeyer flask
used when making a standard
made using an Erlenmeyer flask
An autoclave usually operates at 121 °C and ____________________ psi.
15 psi
Glassware with chips or cracks:
can be repaired with Super Glue
is not a safety hazard
should be discarded
should be used only for noncritical measurements
should be discarded
Urinalysis samples are usually spun in a ____________________.
centrifuge
All of the following are true concerning laboratory balances EXCEPT:
The weighing pan should be cleaned once a day.
They should be checked with known weights at intervals.
They should be installed where they do not get bumped and jarred.
They should not be installed in front of a window that can be opened.
The weighing pan should be cleaned once a day.
used to estimate volumes
beakers or Erlenmeyer flasks
used to determine the alkalinity or acidity of a solution
pH meter
used to store samples and reagents at controlled temperature
Refrigerator
used to weigh laboratory chemicals
analytic balancer
instrument used to sterilize contaminated supplies
autoclave
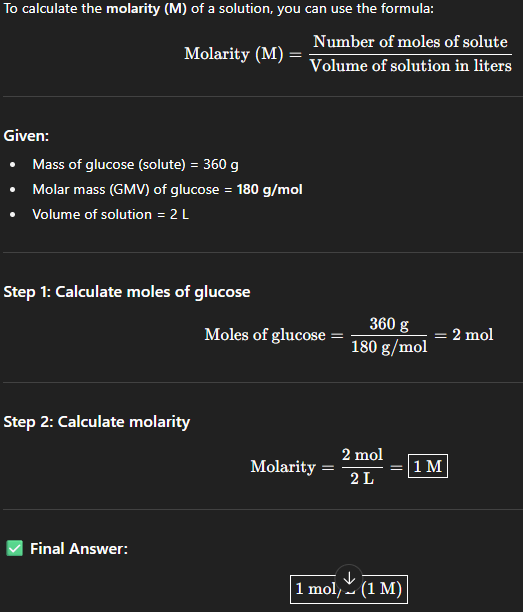
When 360 grams of glucose is added to in water to make final volume as 2L.
What is the molarity of the solution. (GMV of glucose : 180mg)
1M
A 0.85% saline(NaCl) solution is:
8.5 g saline(NaCl) in 100 mL of water
85 g of saline(NaCl) made up to 1,000 mL of water
0.85 g saline(NaCl) made up to 100 mL of water
8.5 mL of saline(NaCl) added to 91.5 mL of water
0.85 g saline(NaCl) made up to 100 mL of water
A solution containing 1 mole of solute per liter of solution is a(n) ______________.
molar solution
In a serial dilution, a sample is diluted a series of times by the same dilution factor.
True
False
true
When 2 g of glucose is added to 100 mL of water, which of the following is true?
It is a w/v solution.
It is a v/v solution.
It is a 2 M solution.
It is a w/w solution.
It is a w/v solution
an expression of concentration and refers to the volume or number of parts of the substance to be diluted in the total volume, or parts, of the final solution.
dilution
What are a series of dilutions in which all dilutions, including or following the first one, are the same….called?
serial dilutions
What is the formula for moles per liter solution?
m = n/v { moles = # of moles / volume (Liters)}
What is the unit for mole per liter?
mol/L
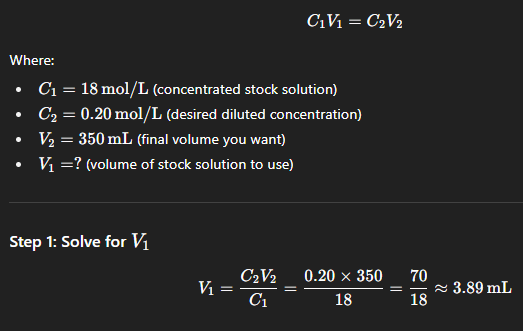
How would you make 350mL of a 0.20mol/L solution of sulphuric acid from a 18mol/L stock solution? solve for the final volume.
3.9mL
The number of moles of solute per kilogram of solvent is called?
Molality
C1V1=C2V2 is the formula for?
Dilution
The gram molecular mass (or weight) of a compound per liter of solution is called
Molarity
What’s the number of osmoles of solute per kilogram of solvent called?
Osmolality
What’s the number of osmoles of solute per liter of solution called?
Osmolarity
-rity correlates to (osmolarity and molarity)
liters
-lity correlates to (osmolity and molality)
kilograms
What are the two types of ware in the lab?
Plastic and glassware