C6.2 Rate of reaction
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What 2 conditions need to be fulfilled for a chemical reaction to occur?
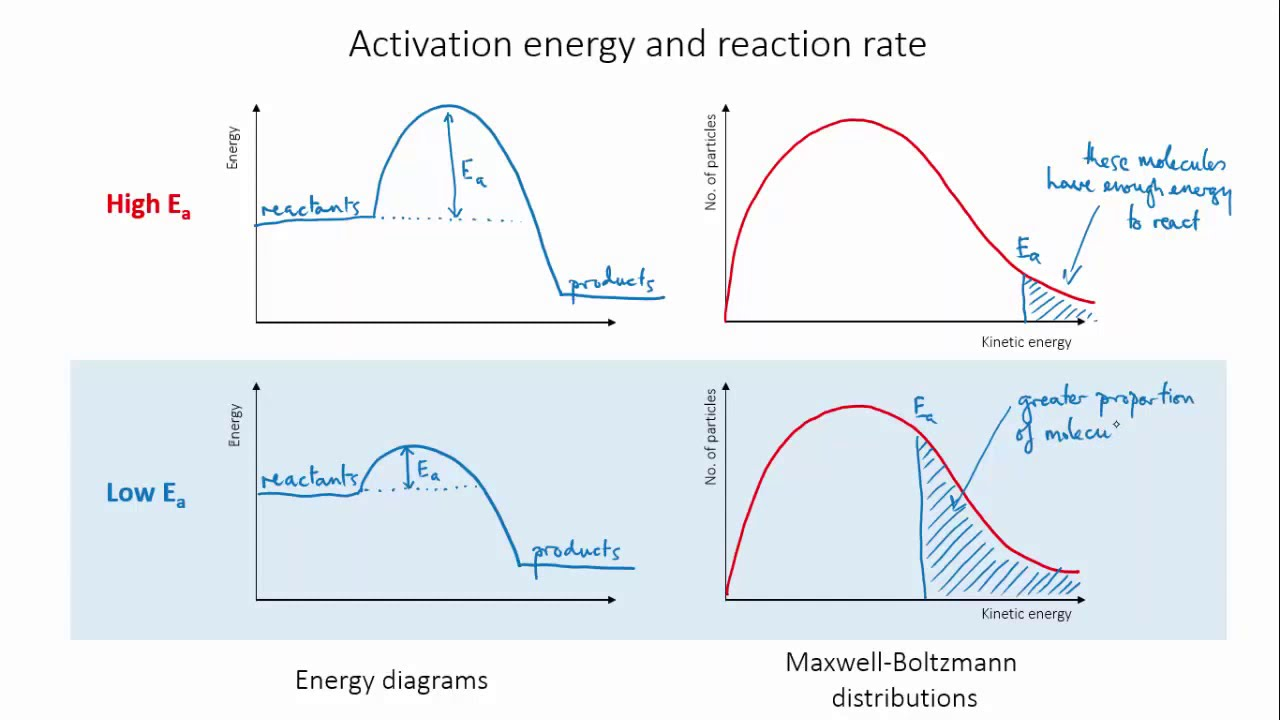
The activation energy of the chemical reaction must be overcome
Molecules of the reactants must undergo a collision in the correct orientation
What is collision theory?
Collision theory states that particles must collide with sufficient energy and in the right orientation for a reation to happen
What factors affect the rate of reaction?
Changing concentration of a solution
Changing pressure of gases
Changing surface area of solids
Changing temperature
Use of a catalyst
Activation energy
What is a catalyst and what does it do?
A substance that increases the rate of a chemical reaction without being used up or changed by the reaction.
It decreases the activation energy of a reaction
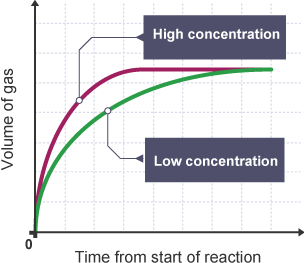
How does changing concentration of a solution affect rate of reaction?
Increasing concentration of particles leads to more frequent collisions between reactant particles
Increases chances of successful collisions, therefore increasing rate of reaction
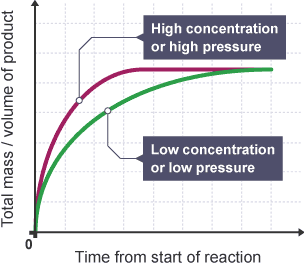
How does changing pressure of gases affect rate of reaction?
Pressure: the force exerted on the walls of a container due to the force of gas particles colliding with the
Increasing gas pressure means gas particles are forced closer together, so they collide with the container walls more often
This leads to more frequent collisions, increasing chances of successful collisions which leads to higher rate of reaction
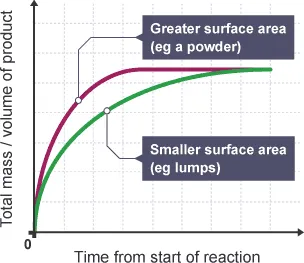
How does changing surface area of solids affect rate of reaction?
If surface area is increased, more particles are exposed to the reactant
This means particles collide more frequently, which leads to more successful collisions meaning a higher rate of reaction
How does changing temperature affect rate of reaction?
When temperature is increased, the kinetic energy of the particles increases leading to more frequent collisions
More frequent collisions leads to more chances of successful collisions, meaning higher rate of reaction
They also have more energy, meaning it is more
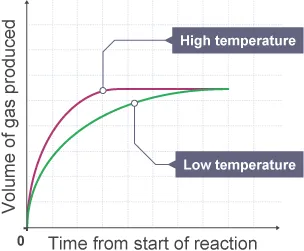
How does the use of catalysts affect rate of reaction?
Catalysts are substances that speed up the rate of reaction. without being used up or changed
With the use of a catalyst, the rate of reaction increases since catalysts help lower activation energy
Lowering activation energy makes it easier to overcome it and have a successful reaction occur faster
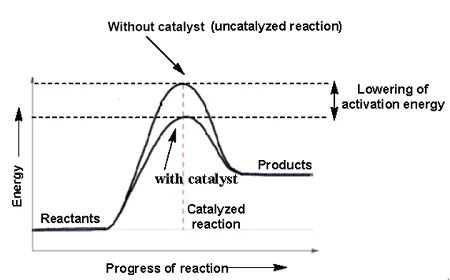
How does the activation energy affect rate of reaction?
Higher activation energy means theres a higher minimum energy to overcome
Particles will be able to reach the minimum less often meaning they can’t collide as often
The rate of reaction lowers