Heat Engine
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
Tc
(1)
Qc
(2)
Engine
(3)
Qh
(4)
Th
(5)
W
(6)
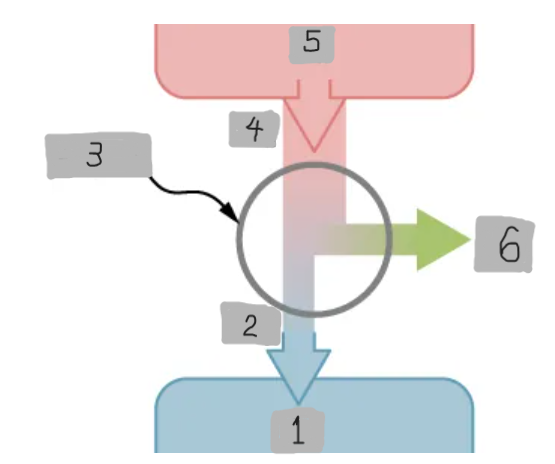
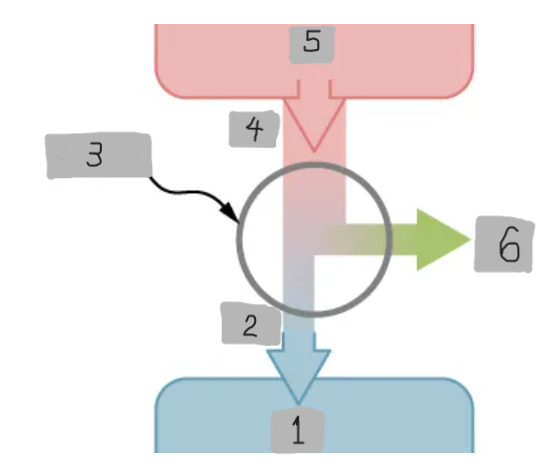
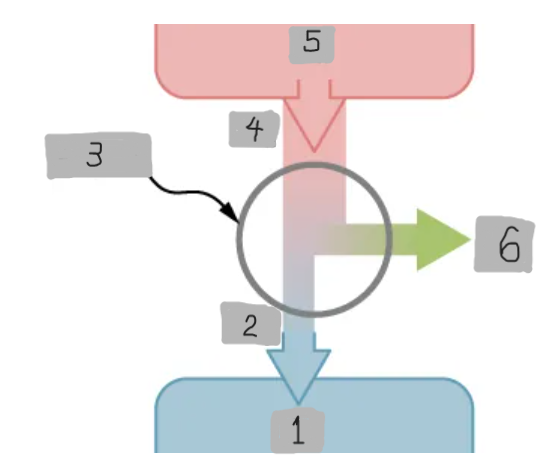
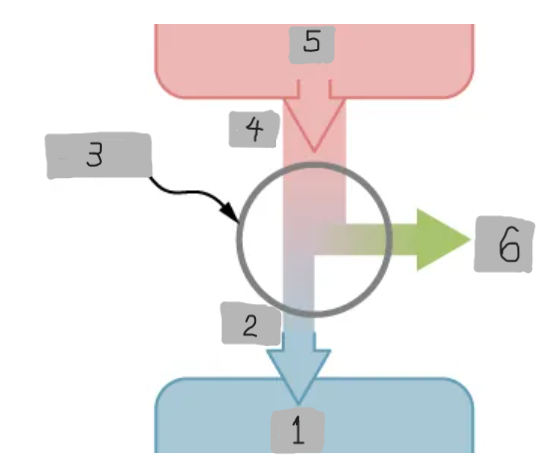
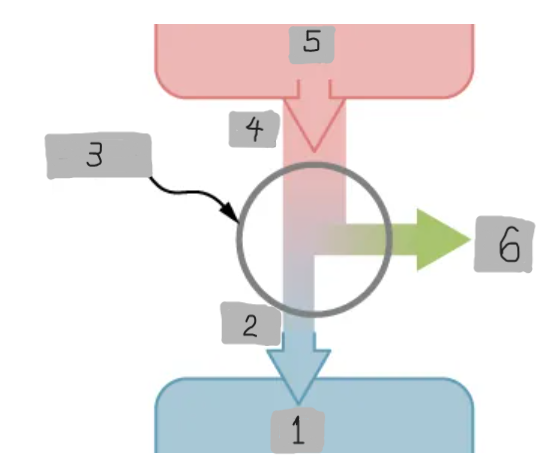
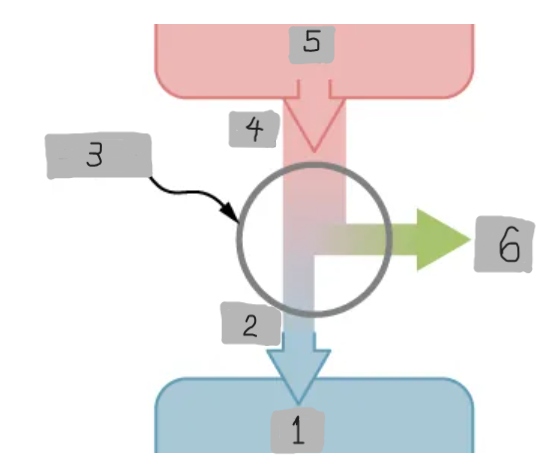
Heat Engine
A device that extracts heat from a high-temperature source, converts part of it into mechanical work, and releases the remaining heat to a cold reservoir.
Heat Reservoir (Th)
The high temperature heat source from which the heat engine absorbs heat Qh.
Cold Reservoir (Tc)
The low-temperature heat sink to which the heat engine releases waste heat Qc.
Working Substance
The material inside a heat engine (e.g., steam gas-air mixture) that absorbs heat, expands or contracts, and performs work during a cycle.
Thermodynamic Cycle
A repeating process in which the working substance returns to its initial state, allowing the engine to operate continuously.
Reversible Heat Engine
An idealized engine with no frictional, thermal, or dissipative losses, operating through quasi-static, reversible processes.
First Law Applied to a Heat Engine
Over one full cycle (ΔEint = 0), the work output is W = Qh - Qc
Efficiency of Heat Engine
The ratio of the useful work output to heat input
e = W/Qh = 1 - Qc/Qh
Mathematical representation of the Efficiency of a Heat Engine
Some energy must be rejected as heat to the cold reservoir; no engine can convert all input heat into work (second law of thermodynamics)
Reason Efficiency < 100%
Clausius Statement (Second Law)
Heat never flows spontaneously from a colder object to a hotter object. This implies that a heat engine requires two reservoirs.
A heat engine must reject waste heat Qc; without a cold reservoir, heat flow stops and no cycle can continue.
Why do we need two reservoirs?
Internal Combustion Engine as a Heat Engine
A gas-air mixture is heated explosively, expands to do work on a piston, and releases exhaust heat to the environment.
Steam Power Plant as a Heat Engine
Steam from boiling water drives a turbine and then dumps heat into cooling water or the air.
Waste Heat
The unavoidable heat Qc, that must be discarded to a cold reservoir, reducing efficiency.
Efficiency and Waste Heat
Higher efficiency → less waste heat (Qc) → lower environmental heat pollution.
W = PΔt
Power to Work Conversion
Qc = W((1/e) - 1)
Minimum Heat Discharge Formula
Raising efficiency decreases Qc, meaning the engine rejects less heat to the environment.
Effect of Increasing Efficiency