Edexcel IAL Biology A-level Topic 1A
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Describe the structure of a water molecule
- One oxygen atom covalently bonded to two hydrogen atoms
- Oxygen is more electronegative than hydrogen which leads to polar bonds and an uneven charge distribution
What is a hydrogen bond?
- A type of strong intermolecular force
- Hydrogen atoms which are directly covalently bonded to a highly electronegative atom (O, N or F) are attracted to highly electronegative atoms in other molecules.
What is a solvent?
Any substance which solutes can dissolve in to form a solution.
State 6 important properties of water
- Acts as a solvent
- Acts as a metabolite
- High surface tension
- High specific heat capacity
- High latent heat of vaporisation
- Strong cohesion and adhesion forces
Why is cohesion useful in biological systems?
Cohesion is the main force supporting columns of water as they are pulled up the xylem in plants. The water molecules stick together as a constant column.
Why is adhesion useful in biological systems?
It allows water to move against the pull of gravity up the xylem.
What are carbohydrates?
Molecules that consist of carbon, hydrogen and oxygen only.
What are monosaccharides?
One individual monomeric sugar unit.
What is a disaccharide?
Two monosaccharides covalently linked by a glycosidic bond.
What is a polysaccharide?
A polymer made of many monosaccharides covalently linked by glycosidic bonds.
What is a glycosidic bond?
A type of bond which joins a monosaccharide to another molecule (usually another monosaccharide to form a disaccharide). It has the following structure:
What type of reaction forms a glycosidic bond?
A condensation reaction
Describe what happens in a condensation reaction
Two molecules are joined together and water is removed.
What type of reaction breaks a glycosidic bond?
A hydrolysis reaction
Describe what happens in a hydrolysis reaction
A molecule is broken apart using water.
Describe the structure of glycogen
Made up of many alpha glucose molecules joined by either alpha 1-4 or alpha 1-6 bonds
- Highly branched
- Compact
Describe the structure of starch
- Made of amylose (joined by alpha 1,4 bonds) and amylopectin (joined by alpha 1,4 and alpha 1-6 bonds)
- Coiled and branched
Why is glycogen useful as a storage molecule in animals?
- It is highly compact
- It is highly branched so it can be broken down by enzymes easily for respiration
- It insoluble
What 3 elements are triglycerides made up of?
Carbon, hydrogen and oxygen.
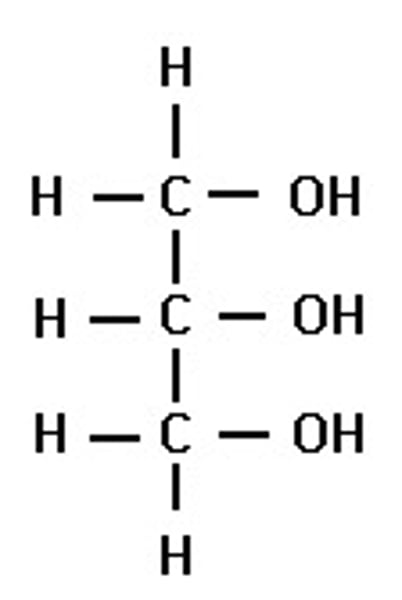
Draw the structure of glycerol
Describe the structure of a triglyceride
- One molecule of glycerol
- Attached to 3 fatty acid chains by ester bonds
- Fatty acid chains may or may not contain double bonds
What type of bond is highlighted below?
An ester bond
What is an ester bond?
A type of covalent bond which is found in triglycerides and phospholipids. Ester bonds join the fatty acid tails to the glycerol molecule and have the following structure:
What is the difference between saturated fatty acids and unsaturated fatty acids?
Unsaturated fatty acids contain C=C double bonds whereas saturated fatty acids only contain C-C single bonds.
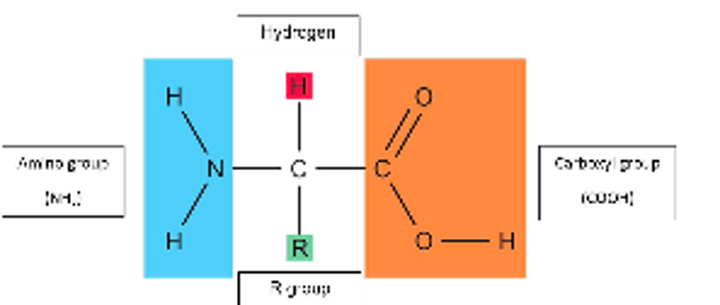
Describe the structure of an amino acid
It contains a carboxyl group, an amino group, a hydrogen atom and a variable R group bonded to a central carbon atom
What is a dipeptide?
Two amino acids joined by a peptide bond
What type of bond joins two amino acids together?
A peptide bond
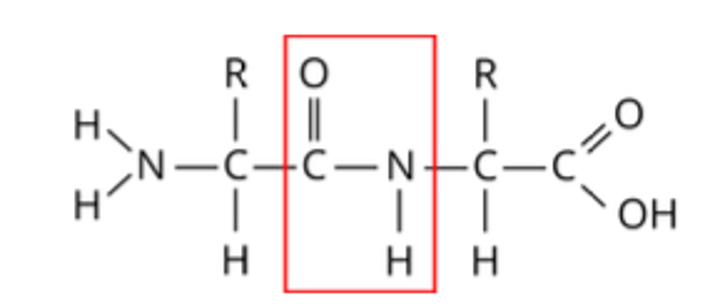
What type of reaction forms a peptide bond?
A condensation reaction
What happens during a condensation reaction?
A bond is formed and a molecule of water is released.
What is a polypeptide?
A polymer made from multiple amino acid monomers joined by peptide bonds in condensation reactions.
What is the primary structure of a protein?
The sequence of amino acids in a polypeptide, held by peptide bonds
What is the secondary structure of a protein?
The local interactions of the polypeptide chain to form 3D structures such as alpha helices and beta pleated sheets. It is held together by hydrogen bonding
State two types of secondary structure in a protein
- Alpha helices
- Beta pleated sheets
What is tertiary structure?
The further coiling of a protein into its functional 3D shape. Held by hydrogen, ionic, and disulphide bonds, and hydrophobic interactions
How does the primary structure affect the tertiary (3D) structure?
R group variations produce different bonds. Sulfur atoms form disulfide bridges, oppositely charged groups form ionic bonds. Hydrogen bonds are always present as they occur between hydrogen and nitrogen/oxygen
What is the quaternary structure of a protein?
-Not always applicable
- Describes the interactions of multiple polypeptide
chains
- Held together by hydrogen, ionic, and disulphide bonds, and hydrophobic interactions
Give one example of a protein with a quaternary structure
Haemoglobin
Give 5 uses of proteins in the body
- Membrane proteins for transport
- Hormones
- Receptors
- Antibodies
- Enzymes
What roles do globular proteins have in the body?
Metabolic roles
What roles do fibrous proteins have in the body?
Structural roles
Describe how the structure of fibrous proteins relates to their function
Long polypeptide chains, folded in parallel. Very little tertiary/quaternary structure aside from cross-linkages for strength. This makes them insoluble, and is useful forproviding structure
Give an example of a fibrous protein and explain how its properties relate to its use
Collagen - Hydrogen and covalent bonds make it very strong. Polypeptide chains form a triple helix which creates fibres. This makes it useful in bones, cartilage and
other connective tissue
Describe how the structure of globular proteins relates to their function
Compact, highly folded with complex tertiary/quaternary structures. Soluble, forms colloids in water. They are useful for hormones, antibodies, etc
Give an example of a globular protein and explain how its properties relate to its use
Haemoglobin - Water-soluble, with a complex quaternary structure. Contains four haem groups that oxygen can bind to. It is therefore used to carry oxygen in the
blood to respiring tissues