Physical Chemistry I Midterm: Quantum Mechanics
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Photoelectric effect
ejection of electrons from metals when they are exposed to ultraviolet radiation
Photoelectric effect demonstrates the particle nature of electromagnetic radiation "photons"
E_ke=hv-Φ
Work function, Φ - energy required to remove electron from metal
experimental characteristics of the photoelectric effect are as follows
· No electrons ejected, regardless of radiation intensity, unless its frequency exceeds a threshold value characteristic of the metal
· The KE of ejected e- increases linearly with the frequency of the incident light but is independent of the intensity of the radiation (KE depends only on frequency)
· Even at low intensities e- are ejected if the frequency is above the threshold value
· Number of e- ejected only depends on frequency of radiation
Black body radiation
all objects emit electromagnetic radiation over a range of frequencies with an intensity that depends on the temperature of the object; radiation emitted by hot objects is discussed in terms of a black body, a body that emits and absorbs electromagnetic radiation as without favoring any wavelengths
Refractive index
the ratio of the velocity of light in a vacuum to its velocity in a specified medium; ni=c/vi where c is the speed of light in a vacuum and vi the speed of light in a material
Snell's Law
n1sinθ1 = n2sinθ2
formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two media, such as water, glass, air, etc.
chromatic aberration
lenses have different focal lengths for different wavelengths meaning lenses focus different colors at different positions causing images with multiple colors to be blurry.
• Shorter wavelengths have shorter focal lengths
• Two lenses can be used to compensate for this
wave-particle duality
recognition that the concepts of particle and wave blend together
particle character of EM radiation
electromagnetic radiation can be thought of as consisting of particles with each particle having an energy hv; these particles are referred to as photons.
What provided evidence for the existence of photons?
experimental evidence from photoelectric effect
What provided evidence of the wave character of particles?
Davisson-Gerner experiment, de Broglie relation,
electron correlation
interaction between electrons in the electronic structure of a quantum system. Correlation energy is a measure of how much the movement of one electron is influenced by the presence of all other electrons
Mean free path
the average distance a molecule travels between collisions
metastable state
an excited state with a long enough lifetime for it to undergo stimulated emission; no allowed transition to relax and emit light
Hamiltonian operator
the operator corresponding to the total energy of the system, the sum of kinetic and potential energies
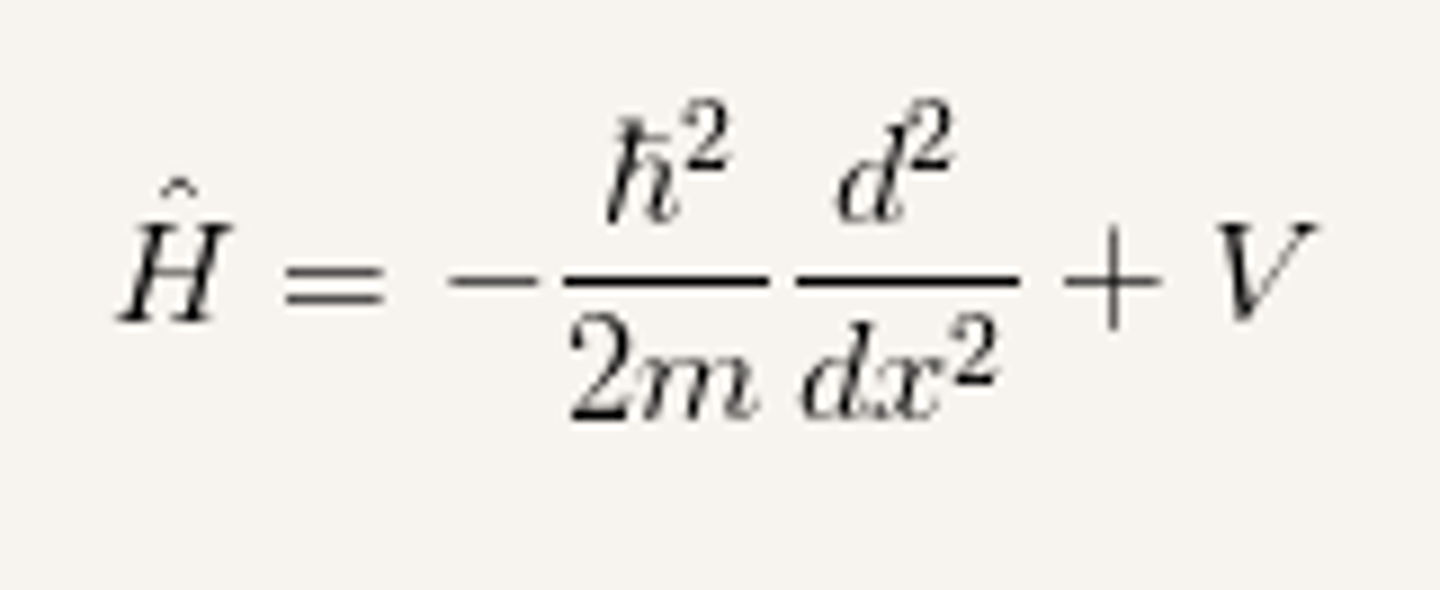
wavefunction (psi)
in quantum mechanics, a particle in a particular state is described by a wavefunction, which contains all the dynamical information about the object in that state such as its position and momentum
Schrodinger equation
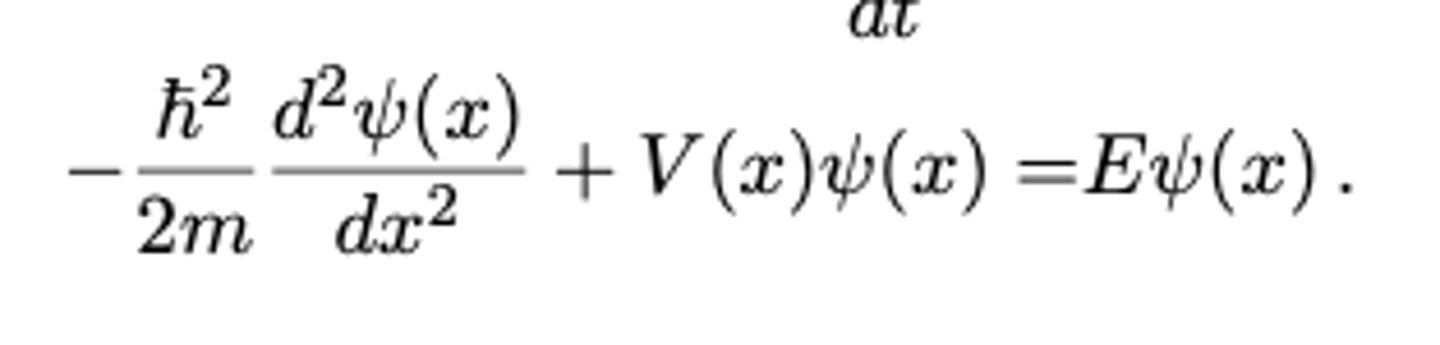
Eigenvalue
(operator)(function)=constant (same function) or Ω ̂Ψ=ωΨ
In an eigenvalue equation, the action of the operator on the function generates the same function, multiplied by a constant. Ψ is said to be an eigenfunction of the operator Ω ̂, and ω is the eigenvalue associated with that function. In H ̂ψ=Eψ, the wavefunction is an eigenfunction to the Hamiltonian and E is the associated eigenvalue.
correspondence principle
as high quantum numbers are reached, the classical result emerges from quantum mechanics
band gap
energy difference between highest valence band and lowest conduction band
thermocouple
two wires, different metals fused at contact point; millivolt potential generated, which varies with temperature
• Used for electronic measurement of temperature
• Can be used in extreme temperatures
• Can be read electronically with A-D converter
basis set
a set of mathematical functions chosen to substitute for the unknown actual wavefunctions is called a basis set
Born-Oppenheimer approximation
supposed that the nuclei, being so much heavier than an electron, move relatively slowly and may be treated as stationary while the electrons move in their field. The nuclei are assumed to be fixed at arbitrary locations, and the Schrödinger equation is then solved for the electrons alone.
electron affinity
the energy released when an electron attaches to a gas phase atom; electron affinity is positive if energy is released when the electron attaches to the atom. That is, implies that electron attachment is exothermic
quantum confinement
describes the restriction of a particle's motion to a small space where its de Broglie wavelength becomes comparable to the physical dimensions of the material
· The particle in a box model illustrates this by showing that confinement leads to discrete, quantized energy levels
Boundary value
outside of the box the wavefunctions must be zero as the particle will not be found in a region where the potential energy is infinite: for x<0 and x=0 and x>L and x=L the wavefunction is equal to zero. These two restrictions are the boundary conditions or constraints on the function
ψ_k (0)=0 and ψ_k (L)=0
Balmer series
A set of spectral lines that appear in the visible light region when a hydrogen atom undergoes a transition from energy levels n>2 to n=2.
Ultraviolet catastrophe
the failed prediction of classical physics that the energy radiated by a blackbody at extremely short wavelengths is extremely large and that the total energy radiated is infinite
Max Planck and Ultraviolet Catastrophe
Max Planck "eliminated" the ultraviolet catastrophe by introducing the concept of quanta, proposing that energy is not continuous but comes in discrete packets (quanta) with specific energies, governed by his new constant (Planck's constant).
morse potential
an interatomic interaction model for the potential energy of a diatomic molecule that more accurately models the higher energy vibrational modes than the harmonic oscillator model
anharmonicity
describes deviation of a system's vibrational frequency from the ideal harmonic oscillator model; motion becomes anharmonic in the sense that the restoring force is no longer proportional to the displacement; energy levels become more closely spaced at high excitations