Chemical Energetics
0.0(0)
Card Sorting
1/15
Earn XP
Description and Tags
Last updated 3:10 PM on 11/5/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
1
New cards
Define the term bond enthalpy
The enthalpy change when one mole of bonds are broken in the gas phase
2
New cards
What is enthalpy change?
A measure of the heat given out or taken in during a reaction
3
New cards
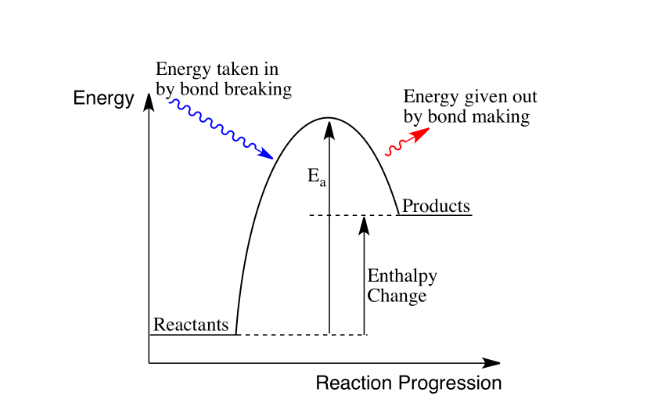
What is an endothermic reaction?
A reaction where heat energy is absorbed
Positive enthalpy change
Positive enthalpy change
4
New cards
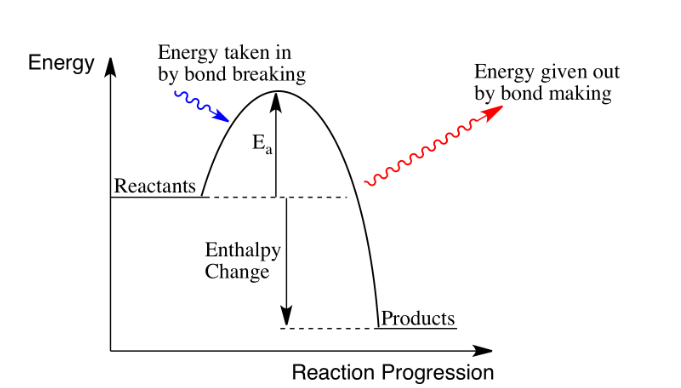
What is an exothermic reaction?
A reaction where heat energy is released
Negative enthalpy change
5
New cards
What is activation energy?
The minimum energy required in order for a reaction to start
6
New cards
What are the standard conditions during a reaction?
* Pressure: 101kPa
* Temperature: 298K
* Concentration: 1 moldm3
* Substance in their most stable state
* Temperature: 298K
* Concentration: 1 moldm3
* Substance in their most stable state
7
New cards
Define the standard enthalpy of combustion
The enthalpy change when one mole of a substance in its standard state burns completely in oxygen under standard conditions
8
New cards
Define standard enthalpy of formation
The enthalpy change when one mole of a substance in its standard state is formed from the pure elements in their standard states under standard conditions
9
New cards
Define Standard Enthalpy of Reaction
The enthalpy change when a reaction takes place in the molar quantities given in a chemical reaction under standard conditions
10
New cards
Define Standard Enthalpy of Neutralisation
The enthalpy change when one mole of water is formed by reacting an acid under standard conditions
11
New cards
Define Standard Enthalpy of Hydration
The enthalpy change that takes place when one mole of gaseous ions dissolves in water
12
New cards
Define Standard Enthalpy of Solution
The enthalpy change that occurs when one mole of an ionic solid dissolves in water
13
New cards
Define the Standard Enthalpy of Atomisation
The enthalpy change when one mole of gaseous atoms are formed from the element in its standard state
14
New cards
What is bond energy?
Energy required to break a mole of covalent bonds
15
New cards
Equation for enthalpy calculations
∆H = m x c x ∆T
16
New cards
What is Hess’ Law?
The enthalpy change of a reaction is independent to the pathway