MICRB 265 topic 5
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
What is the difference between SLP and oxidative phosphorylation?
SLP: metabolic reaction/substrate provides energy to drive ATP production
OxPhos: using the ETC, oxidation (eletron transfer reactions) generate a proton motive force that powers the ATP synthase
What is photophosphorylation?
Capturing light energy to create a proton motive force that drives phosphorylation (of ADP)
Cell resp net reaction and free energy change
Glucos + 6O2 --> 6CO2 + 6H2O
-2895 kJ
What organic molecules can chemoorganotrophs use?
Prefer glucose, but can use dissacharides or other monosaccharides that feed into glycolysis or the CAC
the ATP in glycolysis is made via
SLP (at 1,3-BP to 3-P-glycerate and from PEP to pyruvate)
Glycolysis net reaction
Glucose + 2Pi + 2ADP + 2NAD+ → 2pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
What types of organisms are more likely to use fermentation?
Static, simple organisms (don't need much E or oxygen, just to restore redox balance from NADH made in glycolysis)
CAC is in the
mitochondrial matrix for eukaryotes, the cytoplasm for prokaryotes
Why is the CAC a hub for metabolism?
Organic molecules feed into the CAC to be used for energy, and they exit to provide key anabolic intermediates
Not always aerobic, variations on exact cycle
Pyruvate is converted into ________ to enter CAC
Acetyl Co-A
Pyruvate + NAD+ + CoA --> Acetyl Co-A + NADH + CO2
CAC begins when citrate is made from
acetyl CoA (2C) condensing with oxaloacetate (4C)
NADH vs NADPH
NADPH has phosphate group and tends to work in anabolism rather than ETC
Isocitrate to a-KG step can use NAD+ or NADP+ depending on the species
CAC net reaction
Acetyl CoA + 2 NAD+ + NADP+ + FAD + Pi + ADP + 2H2O --> 2CO2 + CoA + 2NADH + NADPH + FADH2 + ATP + 2H+
(plus one more Co2 and NADH from pyruvate to ACoA)
Two turns per glucose
Reduce lots of electron carriers!
ETC is in
Cytoplasmic membrane for prokaryotes, inner mitochondiral membrane for eukaryotes
Depending on species/conditions, terminal electron acceptors can also be
NO3-, SO42-, multiple
Electrons are passed from ________ in increasing order of reduction potential
NADH/FADH2 --> flavoproteins --> Fe/S proteins -> quinones --> cytochromes --> O2
Iron-sulfur proteins can be in what two types of clusters? Affects what?
Fe2/S4 OR Fe4/S4 --> affects reduction potential and oxidation state of iron, affects how it interacts with the protein
Fe/S clusters are
Metal cofactors used by many different proteins involved in electron transfer (proteins maintain proper chemical environment for ions to give and take e-)
Quinones are
Small membrane-soluble (hphob) molecules (NOT PROTEINS) that can shuttle electrons between carriers (often link Fe/S proteins to cytochromes)
Quinones (ubiquionone) shuttle ____ e- from Fe/S cluster in photosystems to cytochrome C
2 electrons at a time
Cytochromes contain what prosthetic groups?
Heme (iron coordinated within organic molecule)
Different proteins/different heme groups can change reduction potential of cytochrome
_______ are typically the last carrier before the terminal acceptor in the ETC
cytochromes
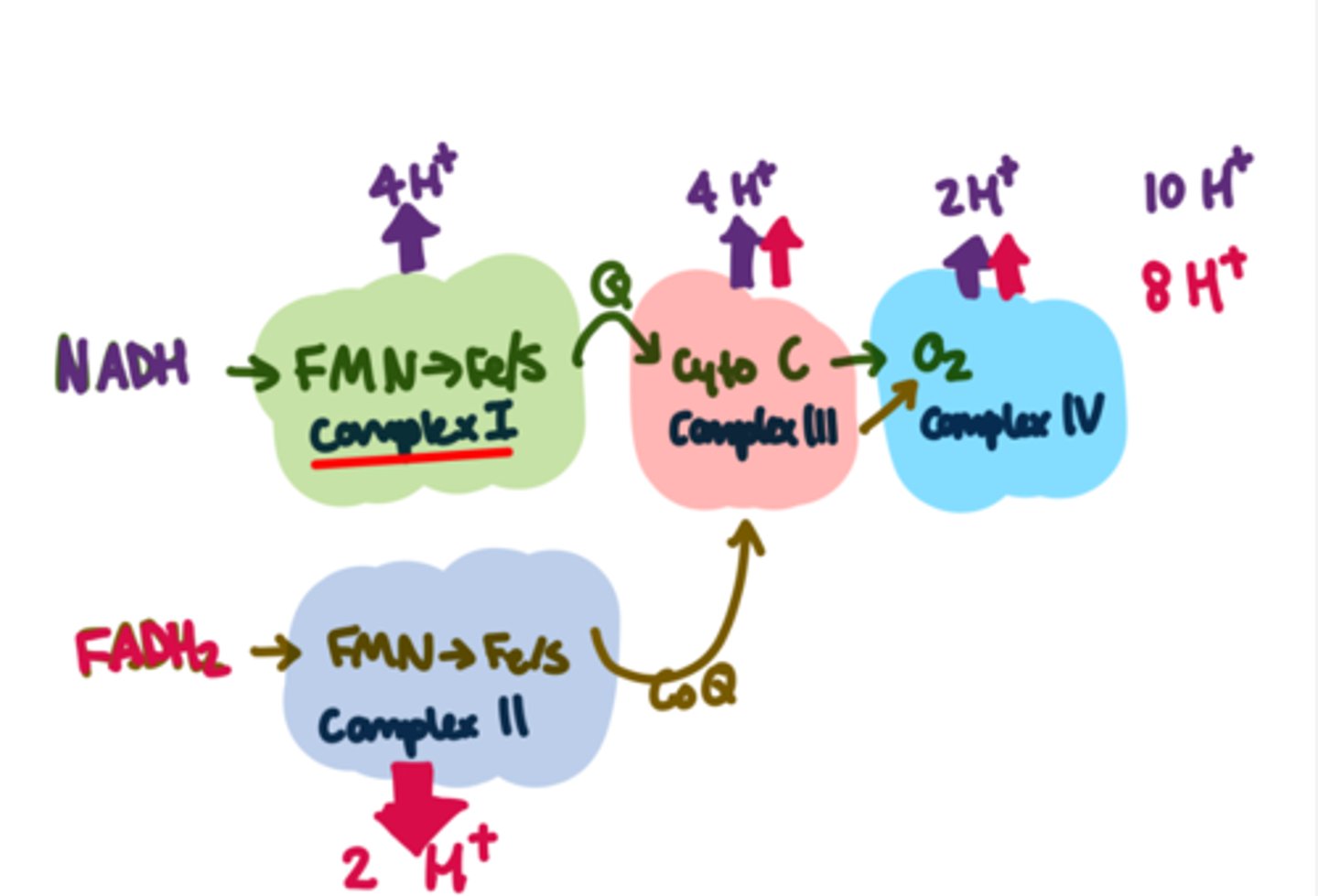
Terminal electron acceptors are _____ quickly and must form _______
Used up (hence we breathe); must form non-toxic product or be removed/processed immediately
NADH produces ______ H+ pumped, FADH2 provides ____
10 (4 at complex I), 8 (2 at complex II)
NADH is a better electron donor (more negative reduction potential) so more energy is released
How many H+ pumped out gives the drive to make one ATP
About 3.3
This is an average number, not the amount actually flowing back in through the ATP synthase
How is the ATP synthase reversible? How do fermenters use this?
Can hydrolyze ATP which provides the energy to pump protons back out and restore the gradient
In fermentation, the ATP synthase is used to create a proton motive force as an energy supply to drive other reactions
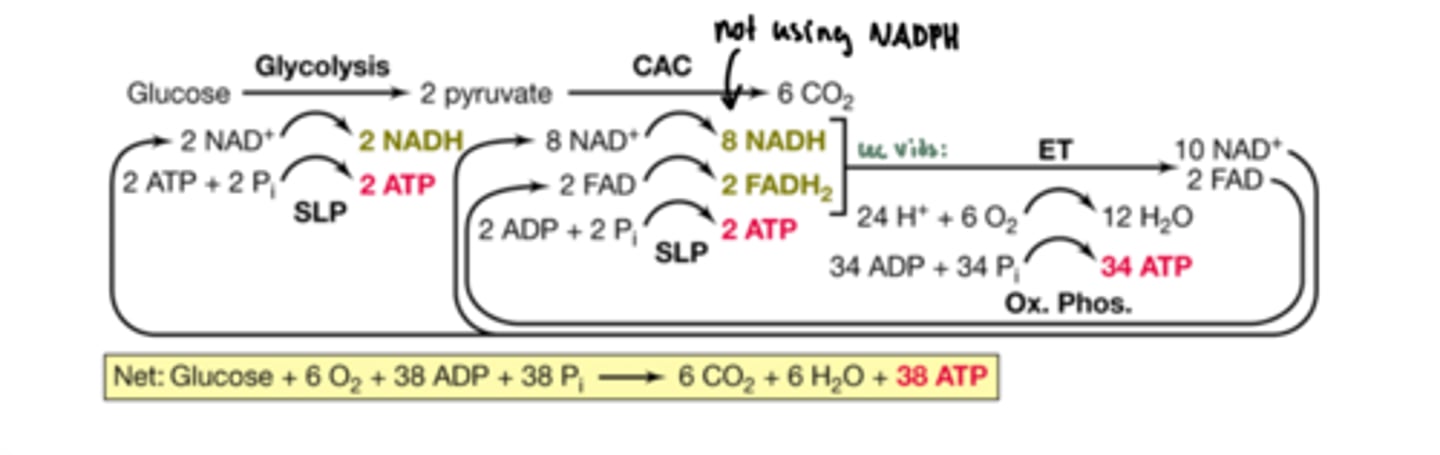
Aerobically one glucose yields about how many ATP
38
Chemoorganotrophs convert fatty acids to ______ using _____
Acetyl CoA, ß oxidation
Chemoorganotrophs convert amino acids to ______
TCA/CAC/Krebs intermediates like pyruvate
Anaerobic terminal electron acceptors in the ETC can be
Nitrate, sulfate
Some microbes can switch between acceptors depending on availability
E. coli is _______robe
Facultative anaerobe -- assembles different ETCs depending on conditions
E. coli can use _______ anaerobically
Nitrate or dimethyl sulfoxide in ETC (not as good as O2 but better than just glycolysis/fermentation), or fermentation as last resort if not available
E. coli don't have _____ in ETC
Complex III, and the final one can change depending on what acceptor to use
Fermentation restores redox balance by
Oxidizing electron carriers (move electrons to fermentation products) and excreting the products
Heterofermentative lactic acid fermenters vs homo
Het = generate mix of lactose and other lactose products - can be useful to avoid lactate/lactic acid fermentationm (low pH can damage cell)
Lactic acid fermentation equation and enzyme
2 pyruvate + 2 NADH --> 2 lactate (excrete) + 2 NAD+
Lactate dehydrogenase
Lactic acid fermentation used for
yogurt, kimchi, sauerkraut
Ethanol fermentation equation and enzyme
1. 2 pyruvate ---(pyruvate decarboxylase)--> 2 acetaldehyde + 2 CO2 (excreted)
2. 2 acetaldehyde + 2 NADH ---(alcohol DH)--> 2 ethanol (excreted) + 2 NAD+
Ethanol fermentation used by
yeast (Saccharomyces cerevisiae) and some bacteria
Ethanol fermentation used to
Make alcoholic drinks
Baking (CO2 raises dough and alcohol will be evaporated off)
Naturally carbonate drinks before forced carbonation
Diversity in fermentation
Fatty acids, amino acids, purines/pyrimidines can all be fermented
Different fermentation products can be make (e.g. mixed acid fermentations - acetate, lactate, succinate, formate, ethanol: prevent accumulation of these metabolites)
Overall common theme is that you generate an E-rich bond that drives ATP synthesis (PEP), donate e- to metabolite (pyruvate/acetaldehyde), and excrete it to get rid of electrons and get redox balance (ethanol/LA)
Only _______ are chemolithotrophs
Prokaryotes (euks need organic food)
Chemolithotrophs can be found
Anywhere with reduced inorganic compounds, but commonly are extremophiles (because no organic sources since other stuff is killed)
Inorganic e- donors include
H2S, H2, Fe2+, NH4+
T/F: chemolithotrophs are anaerobic
F: can be either (many use O2 in ETC)
T/F: chemolithotrophs are mostly autotrophs
True: make their own food by fixing CO2 into organic molecule. REQUIRES LOTS OF NADH (REDUCING POWER) for biosynthetic reactions to allow this
Complex II aka
succinate DH (oxidises succinate to fumarate, generate FADH2)
Common sulfur electron donors (diff E'º and oxidation states)
H2S, elemental S, thiosulfate (S2O3 2-), sulfite (SO3 2-)
Elemental S can be stored in sulfur granules as an energy/electron reservoir)
Note that ACCEPTOR doesn't have to be sulfur just because the food source is (organisms adapt to use what is available, so donors and acceptors can be anything)
Final oxidation product of sulfur donors usually
sulfate
Oxyenic bacteria
Make O2 as biproduct of photosynthesis (e.g. cyanobacteria, algae (eukaryotes)
Anoxyenic bacteria
More ancient than oxygenic phototrophs
Green sulfure bacteria, phototrophic purple bacteria
T/F: phototrophs can be auto or heterotrophs
True, but mostly are autotrophs (use photosynthesis)
Rarely they can get carbon from organic molecules but use light energy to power ETC (photoheterotrophs)
Photosynthetic reaction centres
Complexes of proteins & pigments where electrons are excited and transferred to the ETC
What uses chlorophylls
oxygenic phototrophs
Look like heme groups, but have Mg instead of Fe
What uses bacteriochlorophylls
anoxygenic phototrophs
Look like heme groups, but have Mg instead of Fe
Antenna pigments
Complexes of bacteriochlorophylls/chlorophylls embedded in the membrane that capture light energy and transfer to reaction centre (optimize light capturing)
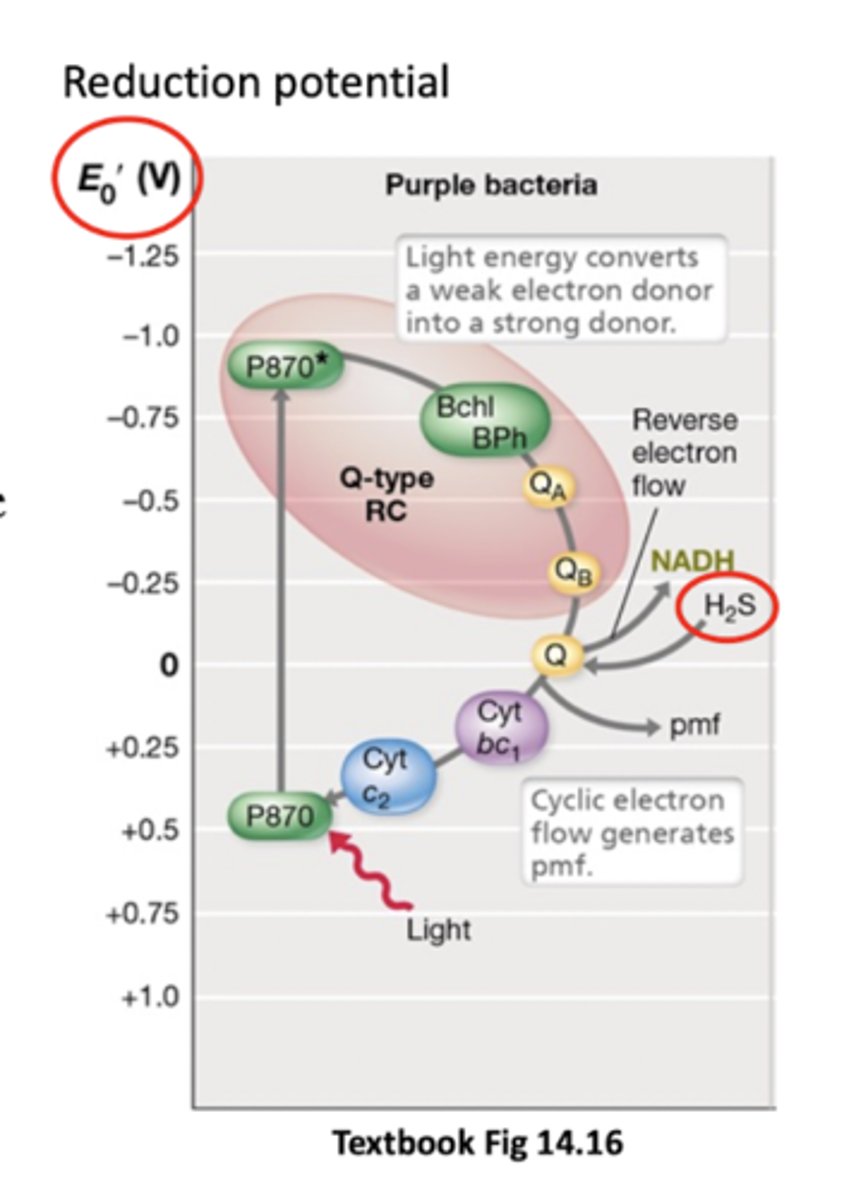
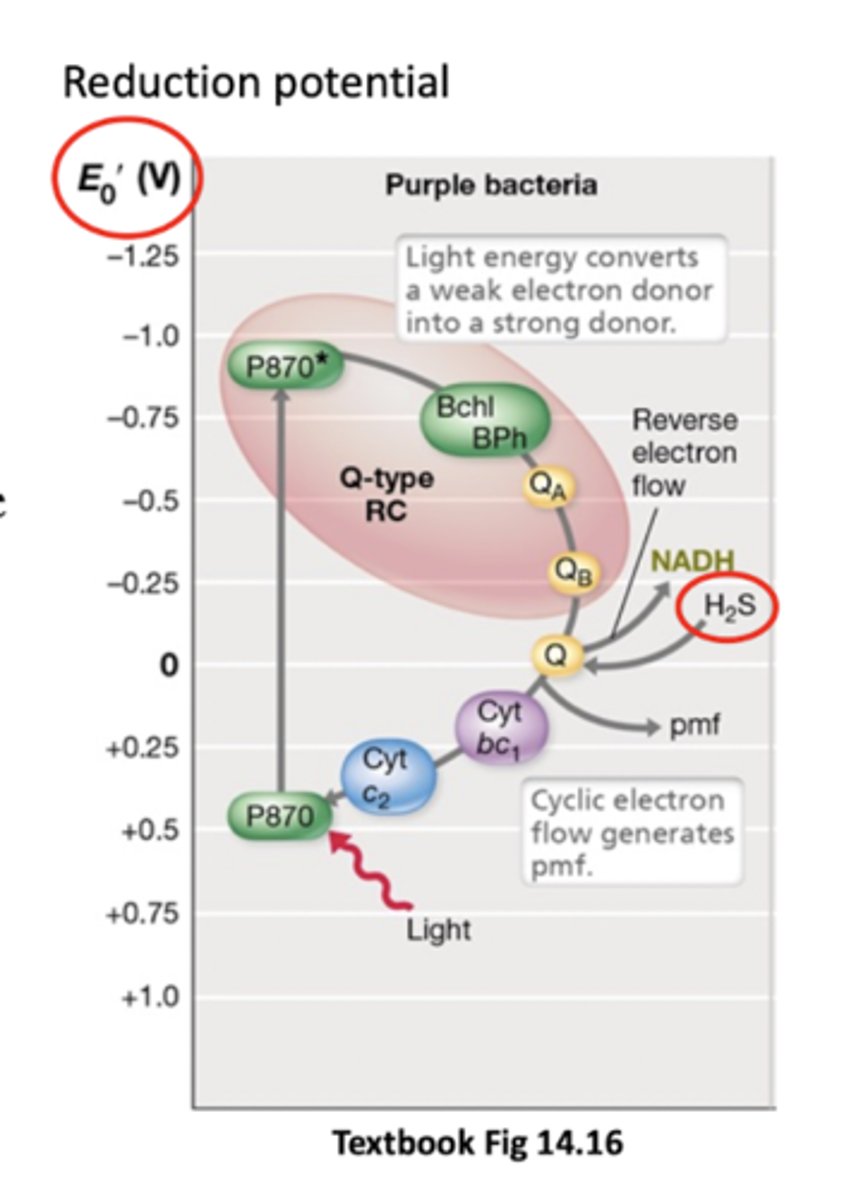
P870
Bacteriochlorophyll in purple bacterior that absorbs light E and goes from weak to strong e- donor
Donates electrons to a quinone where they travel down an ETC and make ATP (P870* then goes back to normal)
Cyclic photophosphorylation
Electrons at the end of the ETC (at cytochrome C2) are taken up by the bacteriochlorophyll (no terminal electron acceptor) and returned back to the first quinone
Happens not in everything but in purple bacteria and some others
Cytochrome C2 can donate backwards because P870 isn't a very good electron donor normally (positive ish Eº')
______ allows different phototrophs to coexist in same habitat
Different pigments with different absorption ranges -- allow organisms to take in a wide spectrum of light from the sun
Pigments can allow you to use light others can't
Q-type reaction centre
Electrons from transferred from reaction centre to quionones
Other anoxygenic bacteria use FeS type
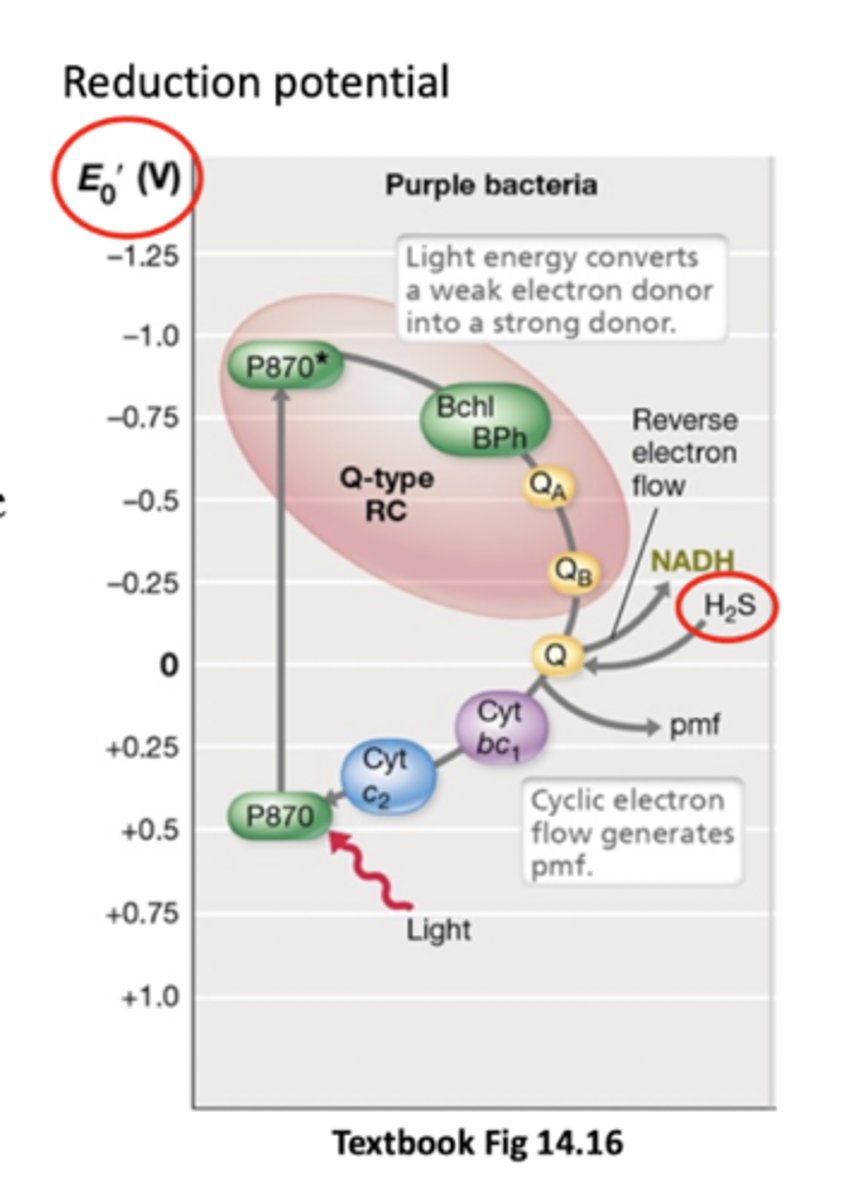
FeS type reaction centres are _____
More efficient at capturing E (Fe/S clusters are stronger electron donors - electrons release more E as they're passed on (more negative reduction potential)
Phototrophs make ______ to use as reducing power in driving metabolism
NADH or NADPH
Electrons for phototrophs usually come from
External donor like H2S (enter quinone pool)
If anoxygenic phototroph lacks an electron donor with negative enough reduction potential to pass external e- to NAD+, what does it do?
It has to use reverse electron transport (use proton motive force to drive electrons in opposite direction of ETC, e.g. from quinones to NAD+) so that it can get a source of reducing power for biosynthesis (NADH)
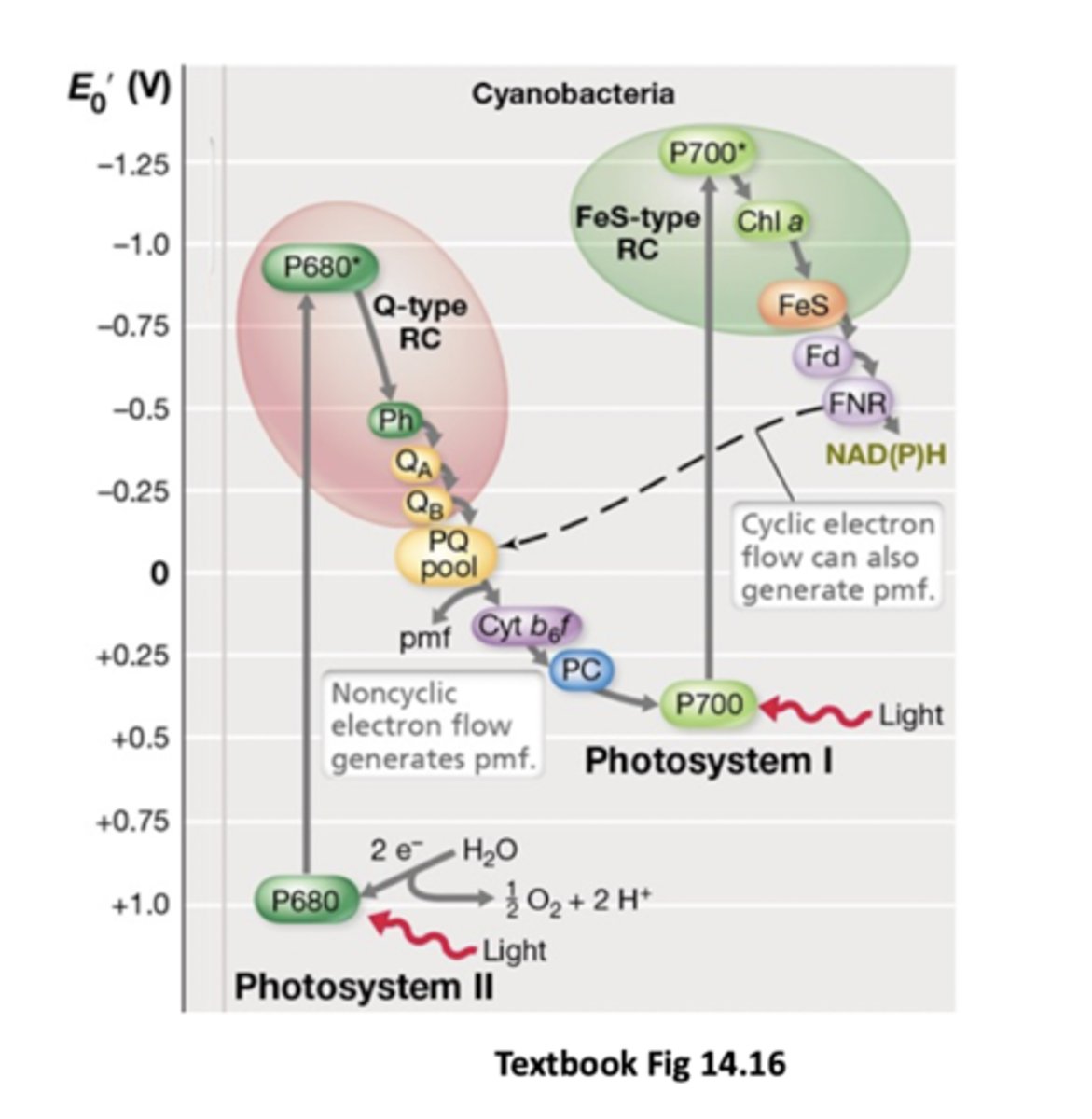
Chloroplasts in eukaryotes evolved from
Cyanobacteria engulfed
PSI aka
P700 - Fe/S type
PSII aka
P680 - Q type
Photosystems are in the
Cytoplasmic membrane for cyanobacteria, thylakoid for eukaryotes (in stacks)
Photosynthesis for oxygenic phototrophs starts at
PSII: excited by light, transfers electrons to quinone pool in system and down ETC (PSI, NADP+)
Pumps out some protons
What happens when light hits PSII (P680)?
P680 donates electrons to the quinone pool and becomes very electropositive
Can take electrons from water to restore electrons (split water into 4H+ + O2)
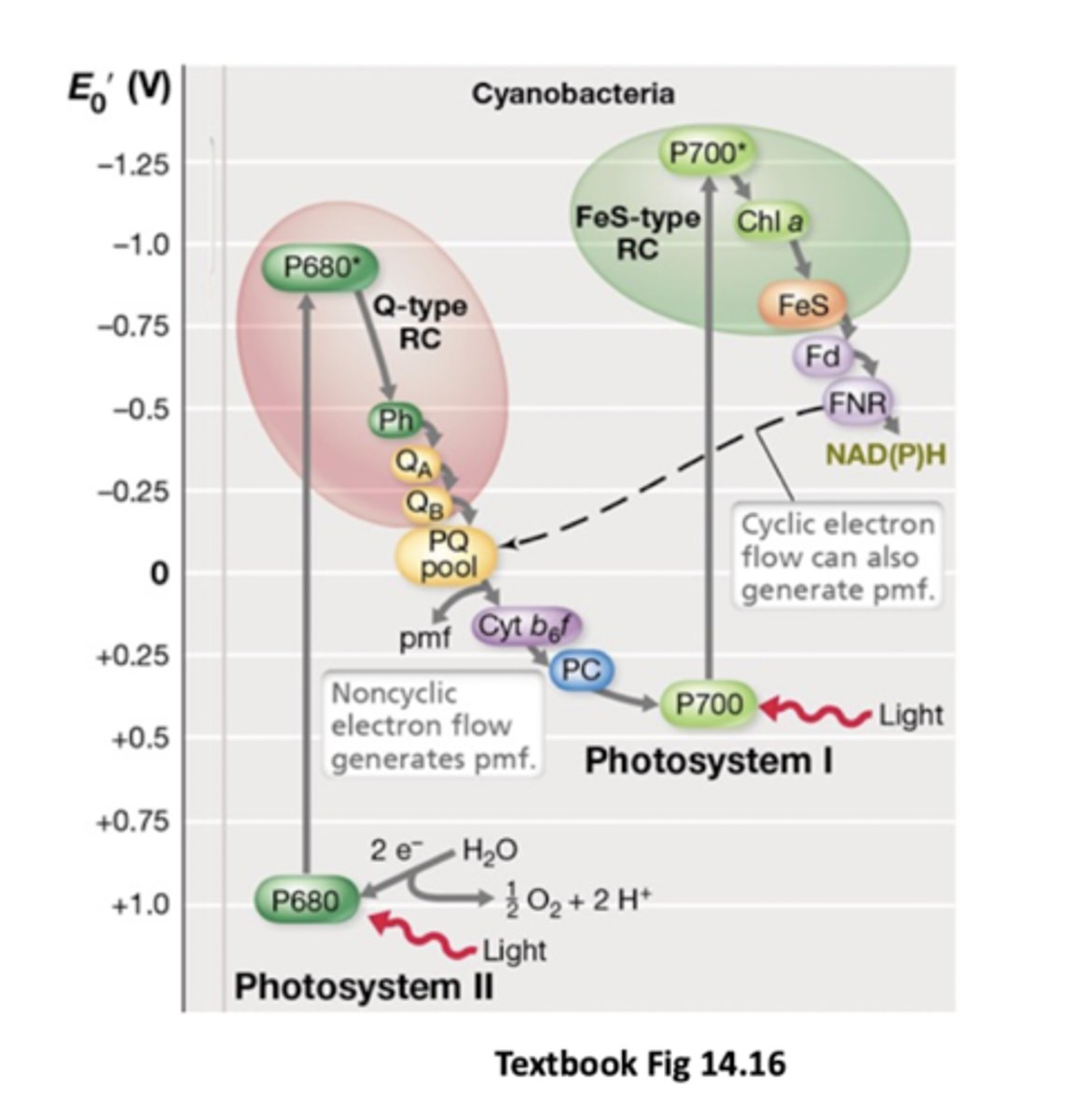
Ultimate electron donor in photosynthesis of oxygenic phototrophs
water (sulfur compounds for anoxgenic bacteria like purple bacteria e.g. H2S)
Order of electron flow in oxygenic photosynthesis once light excites PSII
Quinone pool --> cytochromes --> Fe/S clusters in PSI where it gets reexcited by sunlight, NADP+ --> NADPH --> used to drive biosynthetic reactions like CO2 fixation
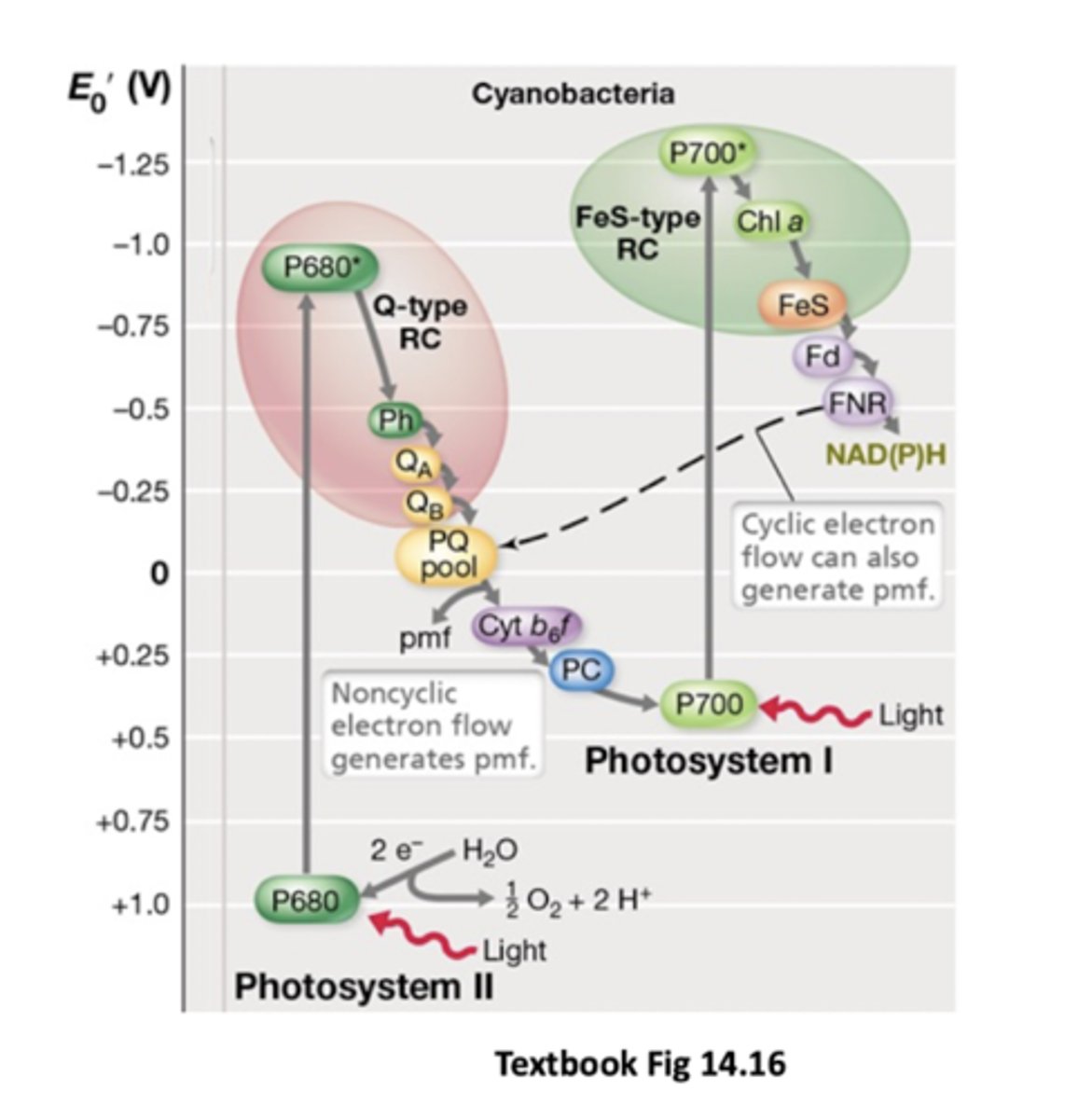
Ultimate electron acceptor in oxygenic photosynthesis
CO2
Do anoxygenic microbes use water as electron source?
No - that means they'd produce oxygen
T/F: most chemolithotrophs and phototrophs are heterotrophs
False: most are autotrophs and need to fix CO2 as organic molecules
For every 6 CO2, the calvin cycle produces
2 glyceraldehyde 3-phosphates that combine into fructose 6-phosphate
First step in calvin cycle (enzyme)
Rubisco: combines CO2 with ribulose 1,5-bisphosphate --> make 3-PG (6C)
Carboxylation
Whole cycle multiplied by 6 (so 6 R15BP and 6CO2)
_____ is the enzyme that does the key carboxylation in the calvin cycle
RuBisCo
6 x 5C + 6 x CO2 --> 12 x 6C (G3P) ------> siphon two off to do what?
Use two glyceraldehyde 3-phosphates to make fructose 6-phosphate --> feeds into glycolysis; highly useful for metabolism
Calvin cycle uses up _____ NADH or NADPH and _____ ATP per 6 CO2 that enter
12, 18
For every 36C into calvin cycle ______ get drawn off for biosynthesis
6 C (2 x GA3P)
T/F: only eukaryotes and archae use calvin cycle
F: phototrophic bactera, most chemolithotrophic bacteria, algae, some archae
Not the only way to fix carbon tho
Effect of N2 triple bond
Very stable, metabolically useless for most organisms even though it is abundant
Needs to be fixed
Nitrogenase
Converts N2 to NH3, which is metabolically useful
N2 + 8H = 2NH3 + H2
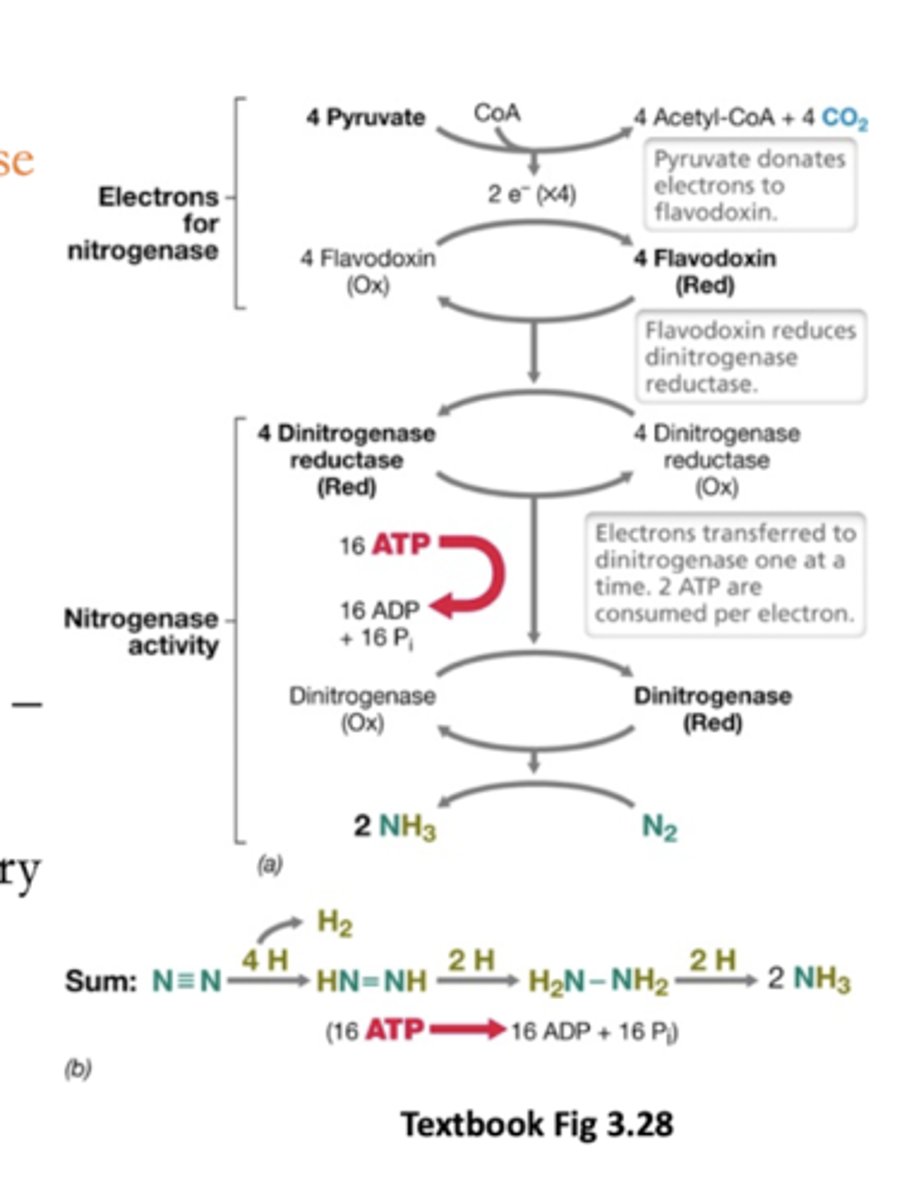
Cells can use NH3 to build
Nitrogen containing molecules like nucleic acids and proteins
Diazotrophs
Bacteria and archea that make nitrogenase and fix N2 into NH3
E.g. cyanobacteria, Rhizobia, some archael metahnotrophs
Nitrogenase is made of which two proteins
Dinitrogenase and dinitrogenase reductase
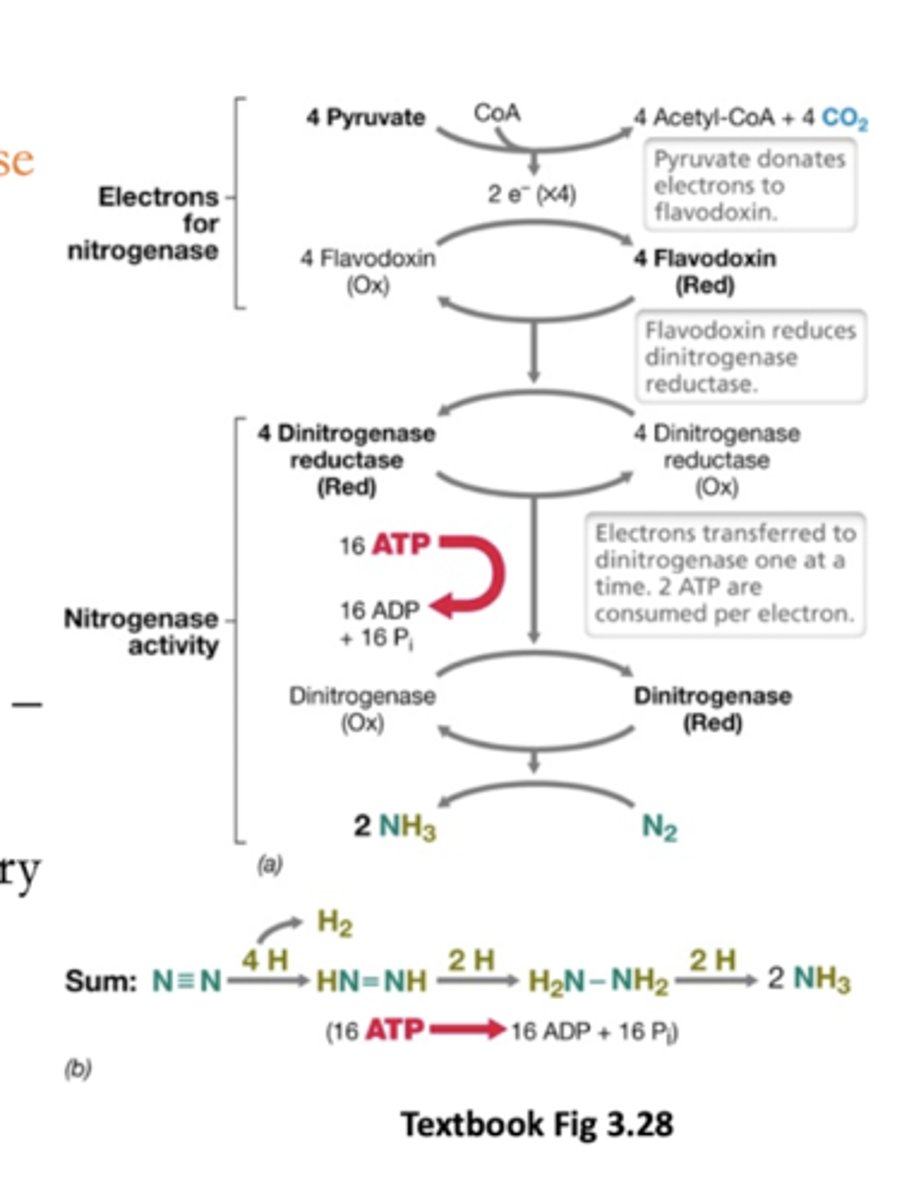
Nitrogenase uses what cofactor
Fe/Mo cofactors
Challenging chemistry = require metals
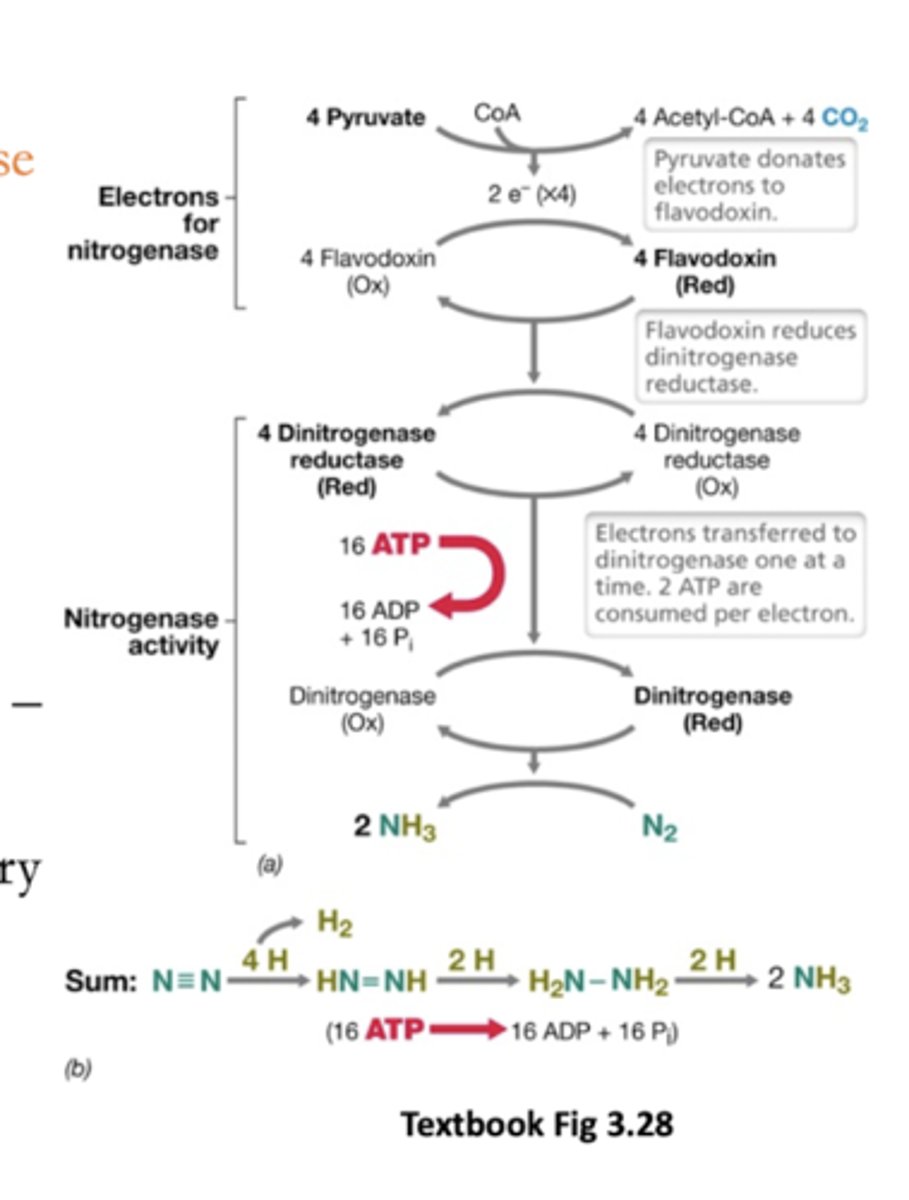
Flow of electrons in the nitrogenase reaction:
From some metabolite (pyruvate) --> Fe/S proteins like flavodoxin --> go to dinitrogen reductase --> dinitrogenase to N2
How many ATP to make 2 NH3 from N2 and 8H+
16 ATP (2 per electron added)
(6 electrons added to Ns plus 2 to H+ -- we don't know why we need to use 8 and produce H2)
Electrons are transferred to dinitrogenase one at a time
How many electrons consumed by nitrogenase
8
Not 6 (reason unknown)
Gluconeogenesis is like
Reverse of glycolysis