Topic 3 - Quantitative chemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
How to workout the % mass of an element in a compound?

What is Avogadro constant?
6.02 X 1023
What is one mole of any substance?
One mole of any substance has 6.02 X 1023 particles - can be atoms, molecules, ion…
One mole of any substance will have a mass in grams that = relative formula mass
How to work out number of moles in a given mass?
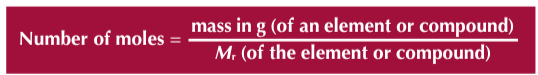
What is conservation of mass in a reaction?
No atoms are destroyed and no atoms are created - mass is conserved?
What can explain if the mass does increase in a reaction?
The reactants is a gas and products are solids or aqueous
Reactant can react with the air in the reaction vessel
What are the explanations if the mass decreases in a reaction?
If the reaction vessel isn’t contained then gas can escape
Why are reactants usually in excess
To make sure that all of the other reactant is used up
What is a limiting reactant?
The reactant that gets used up first
How to calculate moles?
moles = mass/relative formula mass
What is the mass of product called?
Yield of a reaction
How to calculate volume of gas using moles?
Volume of gas = (Mass of gas/ relative formula mass) X 24
What can concentration be defined as?
The amount of a substance in a certain volume of a solution
What is the formula for concentration? (g/dm3 )
Concentration = mass of solute/volume of solvent
What is the formula for concentration? (mol/dm3 )
concenctration = moles of solute/ volume of solvent
How to convert from mol/dm3 to g/dm3 ?
Use the formula mass = moles X M (rearrange it)
What is the atom economy?
The atom economy of a reaction tells you how much of the mass of the reactants is wasted and how much ends up as the desired product.
What is the formula for atom economy?
atom economy = (relative formula mass of desired product/relative formula mass of all reactants) X 100
What is the formula for percentage yield?
(mass of product actually made/ maximum theoretical mass of product) X 100
Why are yields always less than 100%?
Not all reactants react to make a product
There might be side reactions
You lose some product when you separate it form the reaction mixture