Nuclear Decay
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Parent nucleus
any unstable nuclei will spontaneously decay into lighter nuclei. The nucleus that is unstable is called the
Daughter nucleus
the decaying nucleus then leaves behind a
Transmutation
if the decay occurs naturally then it is called a
Artificial transmutation
we can force nuclei to decay and this is called
Types of Decay
alpha decay, beta decay, gamma decay
Alpha Decay - α
occurs when an alpha particle (a Helium nucleus which contains two protons and two neutrons) is emitted from a radioactive nuclei. The alpha particle is the largest of the decay particles and therefore is also relatively slow moving. It has a charge of 2+. When emitting an alpha particle, it carries away with it excess energy
Beta Decay - β
beta decay has two modes which both require the spontaneous change of a nucleon.
• betanegative radiation (an electron) a neutron changes into a proton
• betapositive radiation (a positron) a proton changes into a neutron
In addition to the beta particles released, there are also additional particles that are released to help remove energy from the system. These particles are tiny, neutral and almost massless. They are the neutrino (for β+ decay) and the antineutrino (for β decay)
Gamma Decay - γ
in this decay, rather than reduce the number of nucleons, gamma decay reduces the amount of energy an unstable nucleus has. The gamma ray is a very energetic part of the electromagnetic spectrum that travels at the speed of light. A gamma ray has no charge and no mass. The star on the parent nuclide indicates that the atom is unstable and in an excited state. It needs to lose energy to become more stable
Ionising radiation
Ionising radiation is energy or particles that can remove electrons from atoms. Alpha particles (helium nuclei) are highly positive and ionise air as they pass through. Other examples include beta particles, neutrons, and high-energy electromagnetic radiation like UV rays, X-rays, and gamma rays. Ionising radiation is harmful to living tissue because ionised atoms are highly reactive, leading to unwanted chemical reactions and potentially causing cancer. In contrast, low-energy radiation, such as radio waves, microwaves, and visible light, is non-ionising and poses no significant risk.
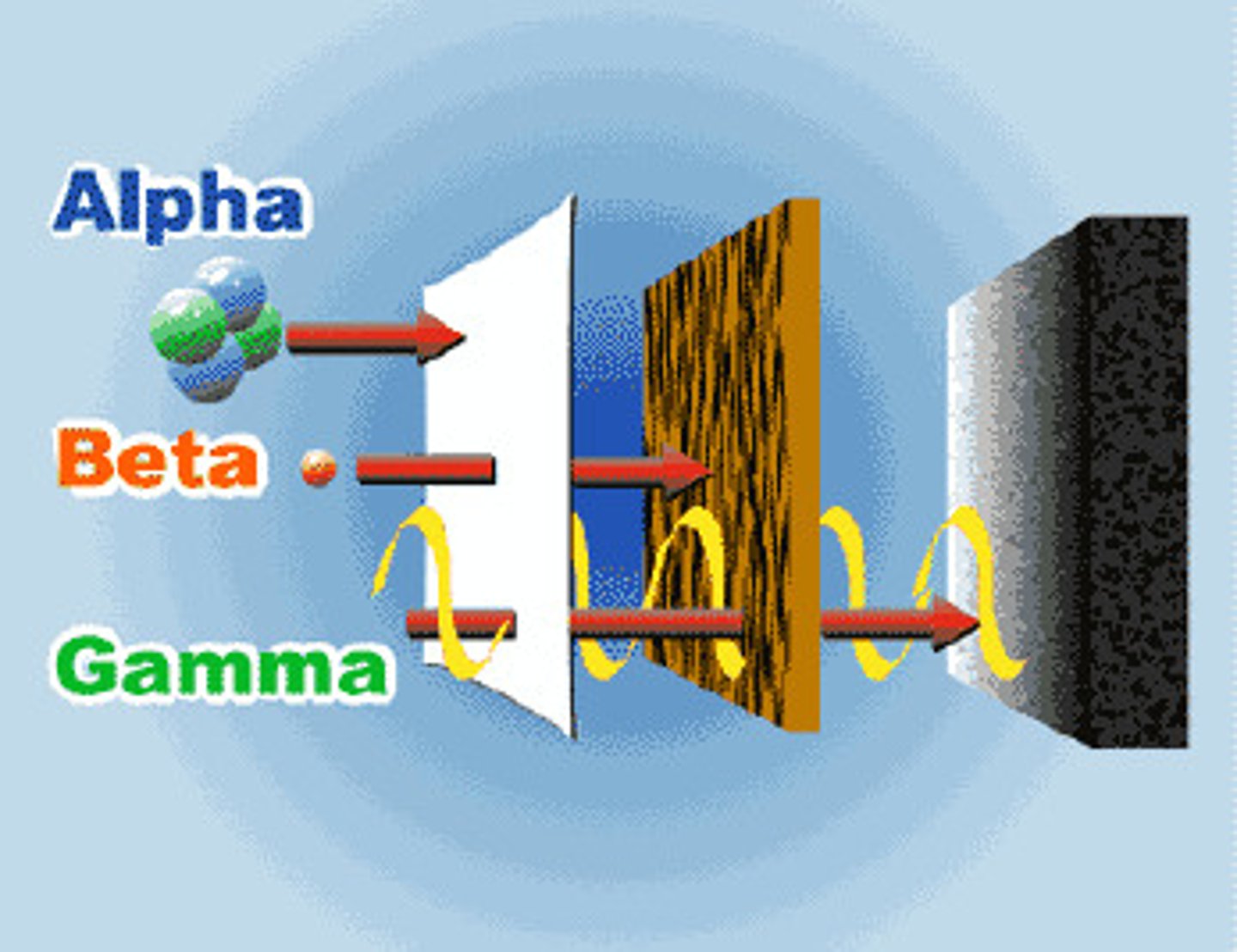
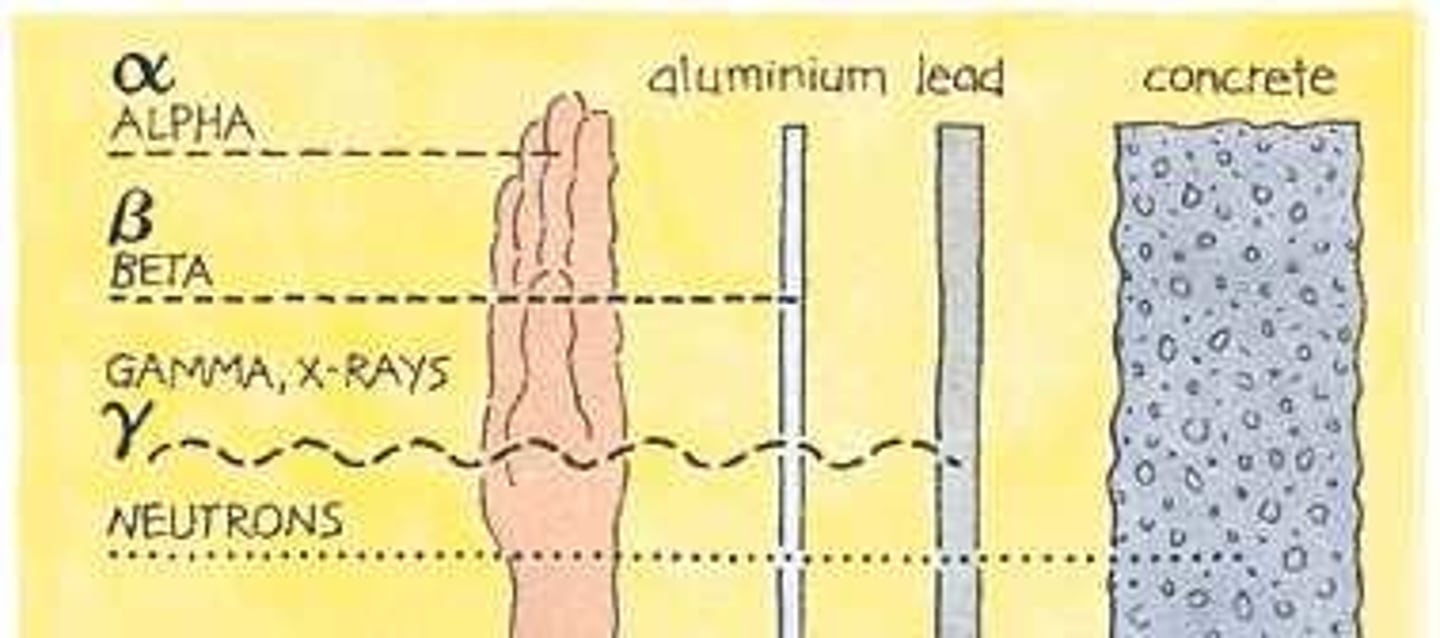
Penetrating ability
Alpha particles will travel a few cm in air, can be stopped by a sheet of paper or skin.
Beta particles will travel a few m in air, can be stopped by 3mm of aluminium.
Gamma rays - will travel a few km in air, can be stopped by thick lead.
Decay series
is a sequence of various radioactive decays by successive daughter nuclei that eventually forms a stable nucleus.
Reason for decay series
Every radioactive nucleus has an excess of energy. In the process of decay, some of the energy is lost in order to obtain a more stable nucleus. Often the daughter nucleus is radioactive, so the process continues.