Serial dilutions and linear regression
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What is a serial dilution
Serial dilutions = are successive dilutions, each with the same dilution factor, where the diluted material of the previous step is used to make the subsequent dilution
- Using serial dilutions, you can create a standard curve for real-time PCR

What should you consider when performing a serial dilution
Consider:
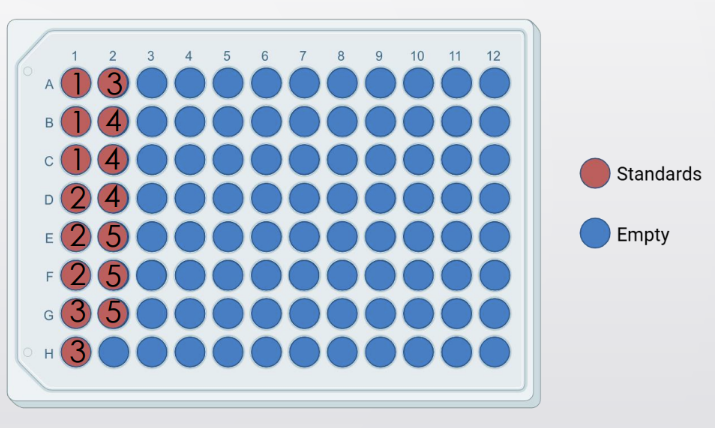
- Each well receives 5uL of standard, control, or patient DNA
- Each standard will be performed in triplicate
- Stock DNA to create the standard curve is at 500,000 copies/uL
What are the steps for a serial dilution
Steps:
1. Decide the volume of each dilution
- Consider how much volume you need from each dilution to accomplish the assay or experiment
o How many uL per well? 5uL
o How many wells? 3 wells
o Total used per dilution? 3*5 = 15 uL (This is NOT enough for each dilution)
2. Account for loss
- Add additional volume to accommodate for loss
- Add 2-3 extra reactions per dilution to account for loss
o 5*5uL = 25uL
- This extra amount is to account for small pipetting loss, but also to account for volumes that will be taken from each dilution to make the next
3. How much do you need of each dilution to make the next dilution?
- Divide your total number by the dilution factor to determine how much of one dilution you need to make the next dilution
o 25uL/10 = 2.5uL
- This is how much you will take from standard 1 to make standard 2
4. Is our total volume truly enough?
- Ask... if I divide my total number (ex: 2.5uL) by my dilution factor (10), and subtract this number from my total number, do I end up with a volume that is adequate to add to the plate? (i.e. cover 3 wells)
o 25uL – 2.5uL = 22.5uL
o Just need 15uL to add to our plate, so this gives an extra 7.5uL to account for pipetting loss
§ More than 50% of what we need, so we do have enough!
25uL will be the TOTAL volume prepared per dilution
Distinguish between the measurements of “copies per reaction” and “copies per microliter” (how do you go from one to the other)
Copies/rxn => copies/uL ==> divide by the volume of the standard that will be added to each well (reaction)
Each reaction (well) receives 5uL, so divide each standard by 5

Know how to prepare a standard curve
Understand the pic

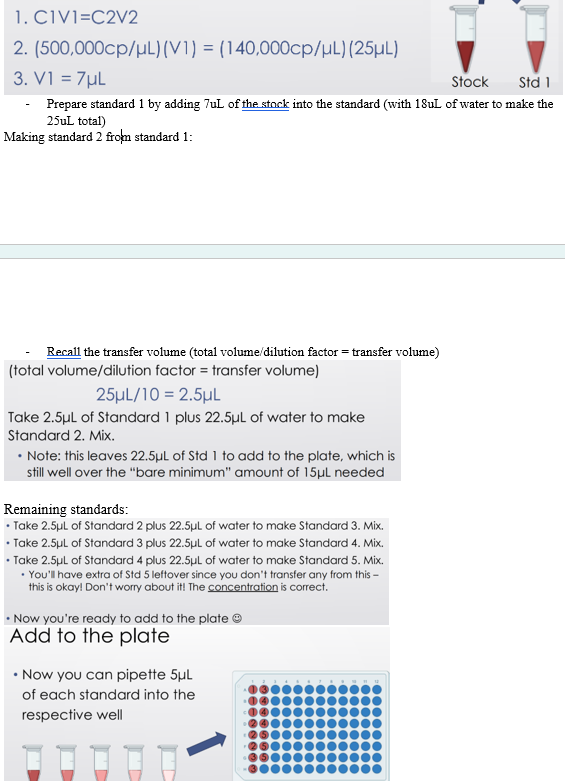
Prepping first standard from stock:
- The total volume needed for each dilution is the amount you’ll make (V2)
PIC
- Prepare standard 1 by adding 7uL of the stock into the standard (with 18uL of water to make the 25uL total)
Making standard 2 from standard 1:
- Recall the transfer volume (total volume/dilution factor = transfer volume)
PIC
Remaining standards:
PIC
What are the components of a straight-line equation?
Y = mx + b x = (Y – b)/m
Y = Ct value
m = slope
x = log copy #
b = y-intercept