Chemistry 3.13- Amino Acids, Proteins and DNA
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
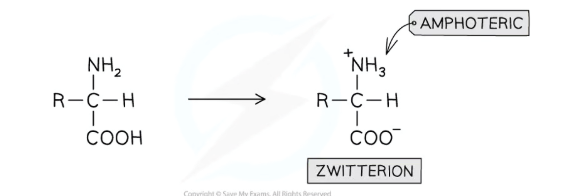
What are zwitterions?
Since amino acids contain a basic amino group and an acidic carboxyl group, an intramolecular neutralisation reaction occurs
This forms a zwitterion, which has both a positive (NH3+) and negative (COO-) charge
2
New cards
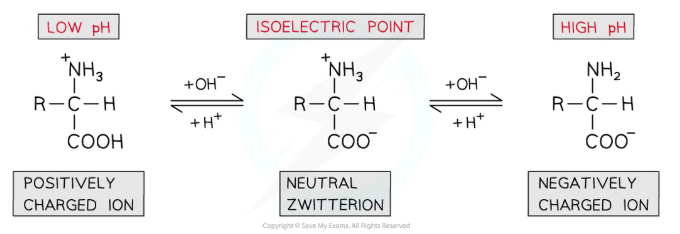
How do amino acids exist at different pHs?
At a low pH, the presence of H+ ions will reprotonate the COO- group in the zwitterion (reforming the COOH), so the amino acid will exist as a cation
At a high pH, the presence of OH- ions will deprotonate the NH3+ group in the zwitterion (reforming the NH2), so the amino acid will exist as an anion
3
New cards
j
m
4
New cards
n
k