Engineering materials
1/258
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
259 Terms
Define crystallography
The science of atoms in solids
Give some information on Nobel prizes in crystallography
Over 25 Nobel prizes on this topic
W. H. Bragg & W. L. Bragg for X-ray work in 1915
Watson, Crick & Wilkins for DNA structure in 1962
Lattices
An infinite array of points (the lattice points) each surrounded by an identical environment
Crystal
Made up of regularly arranged atoms/molecules/ions in a pattern that repeats in multiple dimensions
The unit cell
The volume, mapped out by the vectors in a lattice
They will pack perfectly without empty space
The “right” one is the simplest, smallest one
Describe lattices in vector format
1D: R = ua
2D: R = ua + vb
3D: R = ua + vb +wc
Bravais lattices
An array of discrete points generated by translation operations described by vector operations
Cubic lattice
All sides are the same length
All angles are 90 degrees
Tetragonal lattice
2 of the 3 sides are the same length
All angles are 90 degrees
Orthorhombic lattice
All 3 sides are different lengths
All angles are 90 degrees
Monoclinic lattice
All 3 sides are different length
2 of the 3 angles are 90 degrees
Triclinic lattice
All 3 sides are different lengths
All 3 angles are different
Rhombohedral lattice
All 3 sides are the same length
All 3 angles are the same size but NOT 90 degrees
Hexagonal lattice
Lattice that forms a hexagon
Angles are 60, 90 and 120 degrees
Miller planes
Also called lattice planes
Defined as imaginary planes on which lattice points lie
(Might be good to practice these: they’re not hard but they could come up)
Packing efficiency
The ratio of occupied to unoccupied space in a certain area
Expressed as %
Octahedral hole
A hole that lies between two planar triangles of spheres
Tetrahedral hole
Formed by a planar triangle of touching spheres capped by a single sphere lying in between them
What are some assumptions made when calculating packaging efficiency?
-Ions are incompressible spheres
-Arrangement of Ancona about a cation is stable only of anions avoid contact
-coordination number is as large as possible
Give some info on ionic solids
Generally brittle
High melting points
Most soluble in polar solvents (e.g. water)
Typically form for combinations of elements with large electro negativity differences
Give some info on rock salt structure
4NaCl in unit cell
fcc lattice
octahedral coordination
Cation and anion sites are topologically identical
Give some info on Cesium Chloride
1CsCl in unit cell
Cubic coordination
Adoption by chlorides, bromides and iodides of larger cations
Many metal alloys have a similar structure type
Give some info on ZnS Zinc Blende
4ZnS in unit cell
Tetrahedral coordination
Cation and anion sites are topologically identical
Give some info on single crystals
Very few materials are single crystals
Some examples are silicon (for electronics) and nickel-based superalloys
Give some info on polycrystals
Most metals are polycrystalline
Each separate crystal is called a grain
These have grain boundaries separating the crystals
Stiffness
The resistance. Of a structure to elastic (recoverable) deformation
Stiffness = load/deflection
Units: N/m
Torsional stiffness
Stiffness that exists depending on loading
Stiffness = torque/Angular deflection
Hooke’s Law
Stress is proportional to strain
For simple tension:
Young’s modulus = stress/strain
Modulus
Mechanical property which measures resistance of a material to elastic deformation
The higher the modulus, the more stress is needed to create the same amount of strain
Low modulus materials deflect a lot when bent
High modulus materials deflect very little
What does modulus relate to?
Bonding strength in materials
Also determines natural vibration frequency
low modulus = low frequency
What are the 4 elastic constants in the linear elastic region?
Young’s modulus (E)
Shear modulus (G)
Bulk modulus (K)
Poisson’s ratio (v)
Poisson’s ratios
The change in length divided by original length
Gives a corresponding strain in the axial direction
(Differs from that along the length)
Young’s modulus
Elastic constant for tension
About double the shear modulus
Shear modulus
Elastic constant for shear
Has a relationship with Young’s modulus (v is Poisson’s ratio)
G = E/(2(1+v))
Bulk modulus
Elastic constant for shear
Has a relationship with Young’s modulus (v is Poisson’s ratio)
K = E/(3(1-2v))
Poisson’s ratio of rubber
0.5 for elastomers (rubber)
Thin-walled cylinders expend in radius with no length change only if v = 0.5
Bicycle tyres can be pumped up without getting tighter or looser
Isotropy
When the properties of a material do not depend on the direction in which they are being measured
E.g. glass, ceramics, polymers and metals
Anisotropy
Their properties are dependent upon which direction in the material they are being measured
Give some examples of anisotropic materials
Wood - stiffer along the grain than with it
Fibre-composites are stronger and stiffer parallel to the direction of fibres than perpendicular to them
Cohesive energy
atoms are held together by bonds that behave like springs
Cohesive energy is a measure of the strength of the bonds
What’s the difference between stress and strain?
They are not material properties - they describe a stimulus and response
Stress is applied to a material by loading it
Strain - a change of shape - is its response
What are te stages of selection strategy?
Translation
Screening
Ranking
Documentation
Selection strategy - translation
Translation of design requirements into a prescription for a material, identifying constraints and desired objective
Selection strategy - Screening
Selection out of all materials the fail to meet constraints decided in translation
Selection strategy - Ranking
Ranking of materials decided in screening by their ability to meet the objective
Selection strategy - Documentation
Documentation of candidates that top-ranked during screening, allowing them to be explored in depth
Material index
Objective measures of performance. Can be:
A single material property e.g. tensile strength
A material property group e.g. modulus/density
What 3 concepts are used in the selection procedure?
Performance indices, which isolate the combination of material properties
Materials selection charts
Shape factors: used to quantify the shape of a cross section of beam
What 3 things specify a performance index?
Functional requirements
Geometric parameters
Properties of the material
What can loading on a component usually be broken down into?
Normally some combination of axial tension or compression, bending and torsion
What does p = f(F, G, M) mean?
The equation that describes performance of an object
P = performance
F = functional requirements
G = geometric requirements
M = material properties
f = function of
What do you do if a design includes a single objective that can be limited by multiple constraints?
Each constraint must be evaluated independently
Each constraint is used to obtain a different performance index
How does shape matter in materials selection?
Can modify the resistance to elastic deformation through variation in second moment of area
Will only affect loading situations where I is involved, i.e. bending, torsion or buckling
Efficient shapes use the least material to achieve a given stiffness
Elastic recovery
If load is removed below the yield stress, material returns to original dimensions
Yielding and plastic deformation
Permanent change of shape
Non-recoverable
Metals usually fail by plastic deformation
Creep
Increase of strain at constant stress
Only important for metals at high temperatures
Important for polymers at all temperatures
Fracture
Cracking and separation into two or more parts
Wear
Local removal of material from surfaces in contact and under stress
Tensile testing
Destructive tests which use shaped specimens that concentrate stress in a known gauge length
The sample is put in a tester with one end fixed and the other attached to a moveable cross-head
Fatigue
Failure under repeated (cyclic) loading
Engineering stress
Force over original area
True stress
Force over instantaneous area
What’s the relationship between true stress and engineering stress?
Since original area > instantaneous area, true stress is equal too or larger than engineering stress
Give some info on mechanical failure of ceramics
Only brittle fracture occurs with ceramics
No/very little plastic deformation occurs
Why doesn’t plastic deformation occur in ceramics?
At room temperature most ceramics materials suffer fracture before the onset of plastic deformation
Give some info on dislocations movement in crystalline ceramics
Very difficult because of ionic bonding
Very few slip systems in which the dislocations can move
Covalent bonds are relatively strong and need to be broken
Give some info on dislocations movement in non-crystalline ceramics
Plastic deformation does not occur by dislocation motion - no regular atomic structure
Occurs by viscous flow instead
Viscosity of glasses is very high and they tend not to deform before failing
Types of failure in ceramics
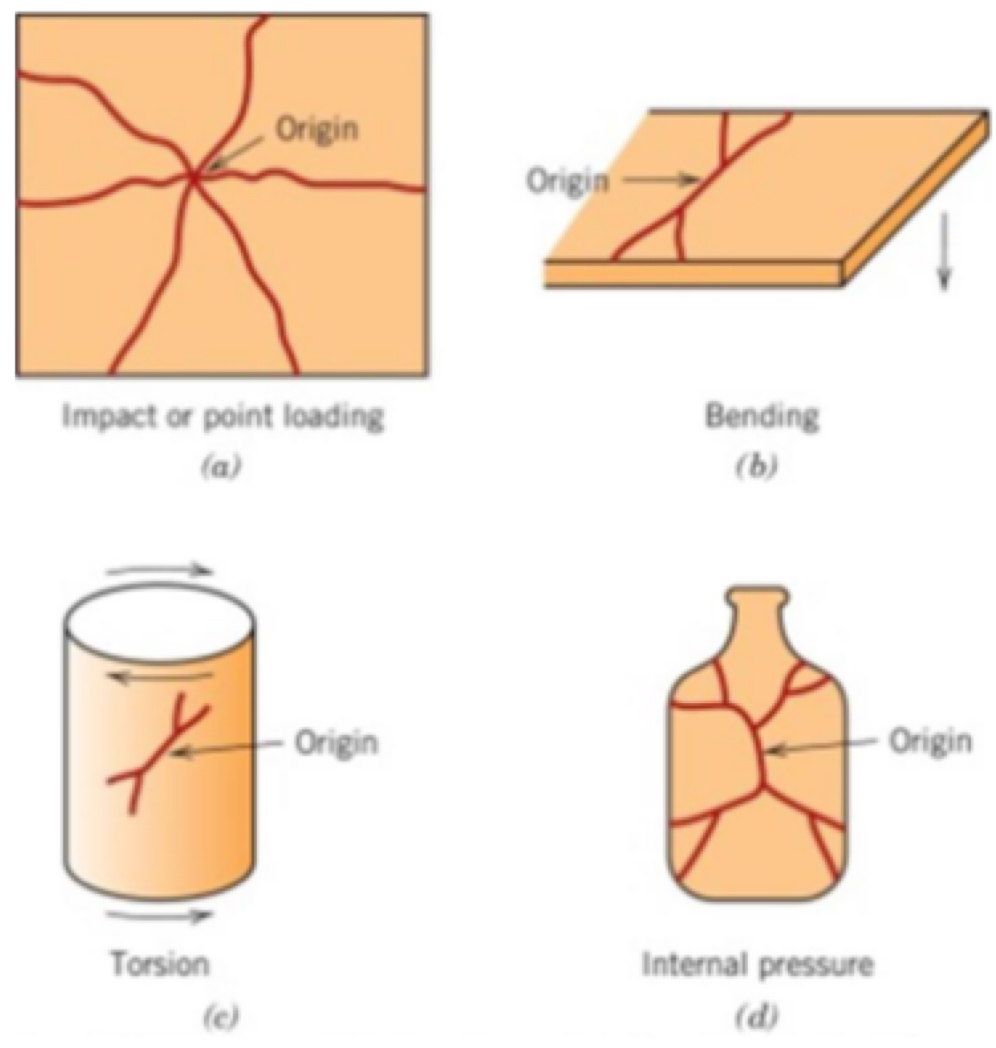
how strong are ceramics?
Ceramics are generally very tough/strong materials
Give some info on failure of ceramics
Ceramics fail because they have many surface flaws
Often, the ‘worst flaw’ propagates to failure
However, compressive failure can result by collapse of crush band of fragmented material
Why don’t we test ceramics in tension?
Difficult to prepare samples with required geometry
Difficult to grip samples without fracturing them
Ceramics show little/no plastic deformation before failing
How is tensile strength measured in ceramics?
Bend tests are used e.g. three-point
The top surface is in compression and the bottom surface is in tension
Please compare three and four-point bend tests
Four-point tests have constant bending moment between central supports
Three-point tests have high stresses at central loading point - can damage specimens
Are ceramics stronger in tension or compression?
Compression
Compressive strength = 10 x tensile strength
How do engineers use ceramics in practical applications?
Designs need to keep ceramics under compressive loading
E.g. reinforced concrete uses steel bars pre-stressed in tension to keep the surrounding concrete in compression
Which is more likely to break: a clay pot or a coffee mug?
The clay pot
Coffee mugs are normally glazed and therefore less porous
Give some info on mechanical failure of polymers
Polymers are similar to metals in that they can fail through fracture or plastic deformation
This is dependent on the polymer
Give some info on mechanical properties of polymers
Fracture strengths of polymers - 10% of those for metals
Deformation strains for polymers > 1000%
For most metals, deformation strains < 10%
Give some info on a non-cross linked polymer structure
No regular repeating pattern of polymer chains
Results in a glassy or amorphous structure
Give some info on a partly crystalline polymer structure
Regions in which polymer chains line and and register
Forms crystalline patches
Give some info on a slightly cross-linked polymer structure
Occasional cross-linking allowing the polymer to stretch
Typical of elastomers
Give some info on a heavily cross-linked polymer structure
Heavily cross-linked polymers exhibit chain sliding
Typical of epoxy
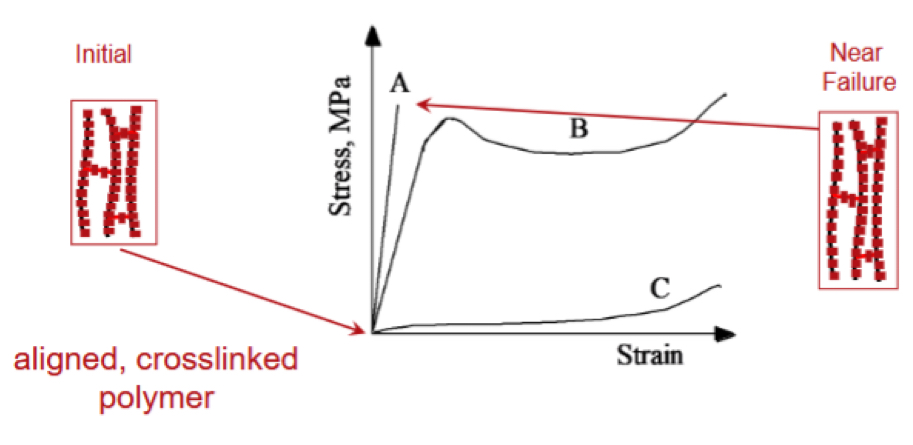
Give some info on the different polymers shown on this stress-strain curve
Polymer A is a brittle and is either heavily cross-linked or networked polymer
Only allows elastic deformation before failure
Only polymer B shows any significant plastic deformation
Give some info on the effect of temperature on tensile strength
As temperature increases the strength of most materials decreases
Strength decrease with temperature depends on material and temperature change
Polymers typically are very temperature sensitive
How does temperature increase effect temperature strength in polymers?
-decrease in the elastic modulus
-a reduction in tensile strength
-an increase in ductility
How are polymers normally strengthened?
Dislocations do not play a role in the strength of non-crystalline solids
Strengthening polymers can be done through blending, drawing, cross-linking, and by reinforcement
How does plastic deformation occur?
When large numbers of dislocations move and multiply
Results in macroscopic deformation
If we want to increase yield and tensile strength of a material, what do we do?
We need to introduce a mechanism which prohibits the mobility of these dislocations
Crystalline imperfections
Defects in metals and ceramics which prevent materials from achieving their ideal strength
Name some common examples of crystalline imperfections
Vacancies
Dislocations
Grain boundaries
Solute atoms on interstitial and substitutional sites
Dislocation
An extra half-plane of atoms in the crystal
Dislocations distort the lattice and make metals soft and ductile
Grain boundaries
Formed when different oriented crystals meet
The individual crystals are called grains, the meeting surfaces are grain boundaries
Made by cutting, slipping and rejoining bonds across a slip plane
Why is grain size important?
The size of the grains of our material can have an influence on the strength of the material
Typically, the smaller th egrain size the stronger the material
Grain
An individual crystal in a polycrystalline metal or ceramic
Grain boundary
The interface separating two adjoining grains with different crystallographic orientations
Grain size
The average grain diameter as determined from a random cross section
Oi, give us the Hall-Petch equation
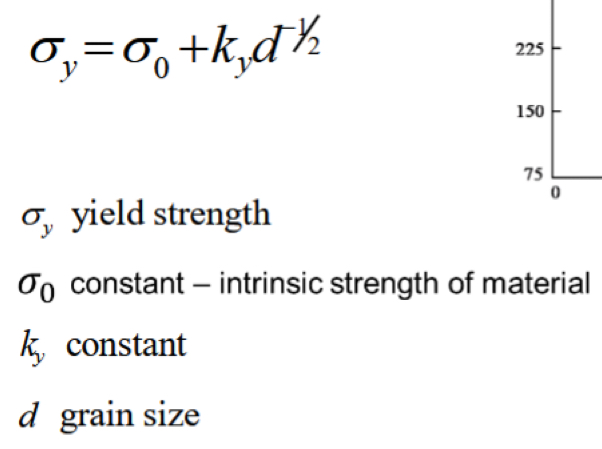
How can we control grain size?
Control during processing (casting, rolling, extrusion, heat treatment)
What are the different types of solid solutions?