Aldehydes and Ketones
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What are carbonyls?
Compounds with a C=O bond.
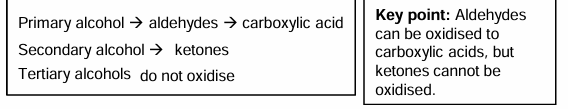
Aldehydes, ketones or carboxylic acids.
Aldehydes and carboxylic acids have it at end of chain whereas ketones have it in the middle.
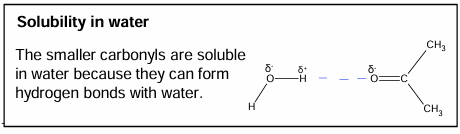
Intermolecular forces in aldehydes and ketones

Solubility in water

Why is C=O polar?
Because O is more electronegative than carbon.
The positive carbon atom attracts nucleophiles.
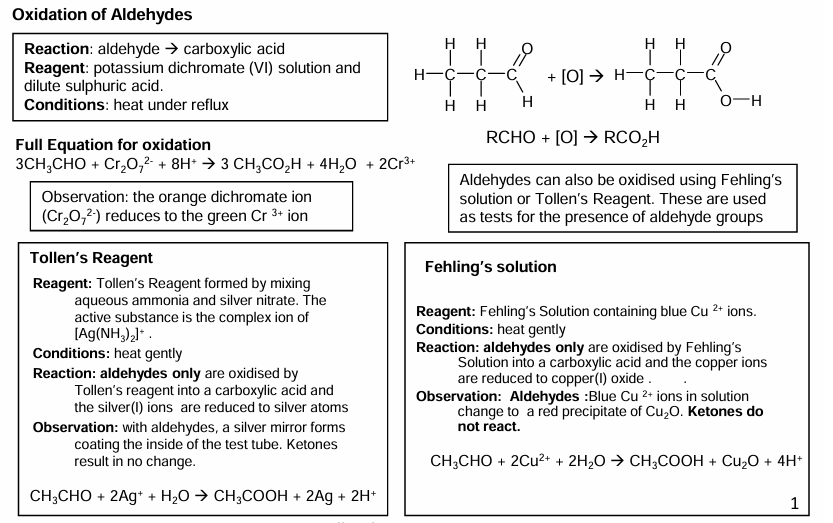
Oxidation reactions
Kr2CrO7 is an oxidising agent that causes alcohols and aldehydes to oxidise.

What is produced when heated under reflux?
JUST aldehydes
Oxidation of aldehydes

Reduction of carbonyls
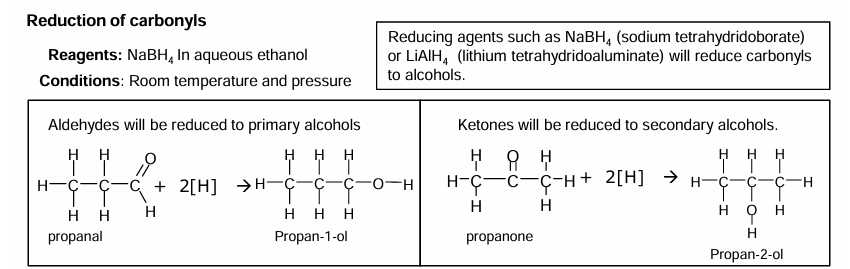

Mechanism for reduction mechanism: Nucleophilic addition
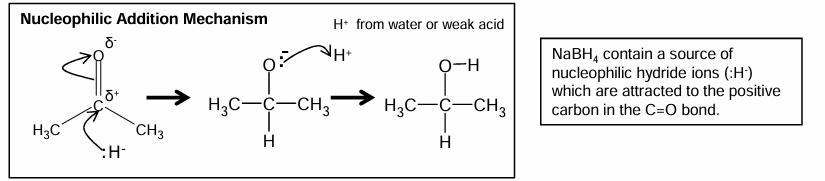

Catalytic hydrogenation
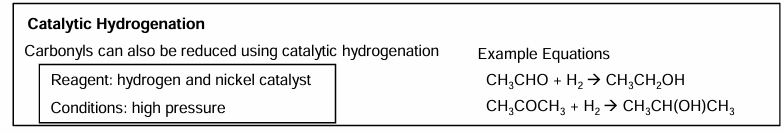

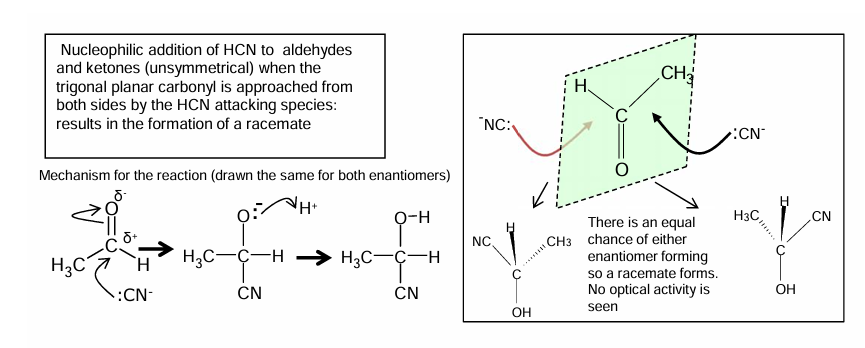
Addition of hydrogen cyanide to carbonyls to form hydroxy nitriles: conditions

Mechanism for addition of hydrogen cyanide to carbonyls to form hydroxy nitriles

How a racemic mixture can be formed when HCN is added to aldehydes and ketones